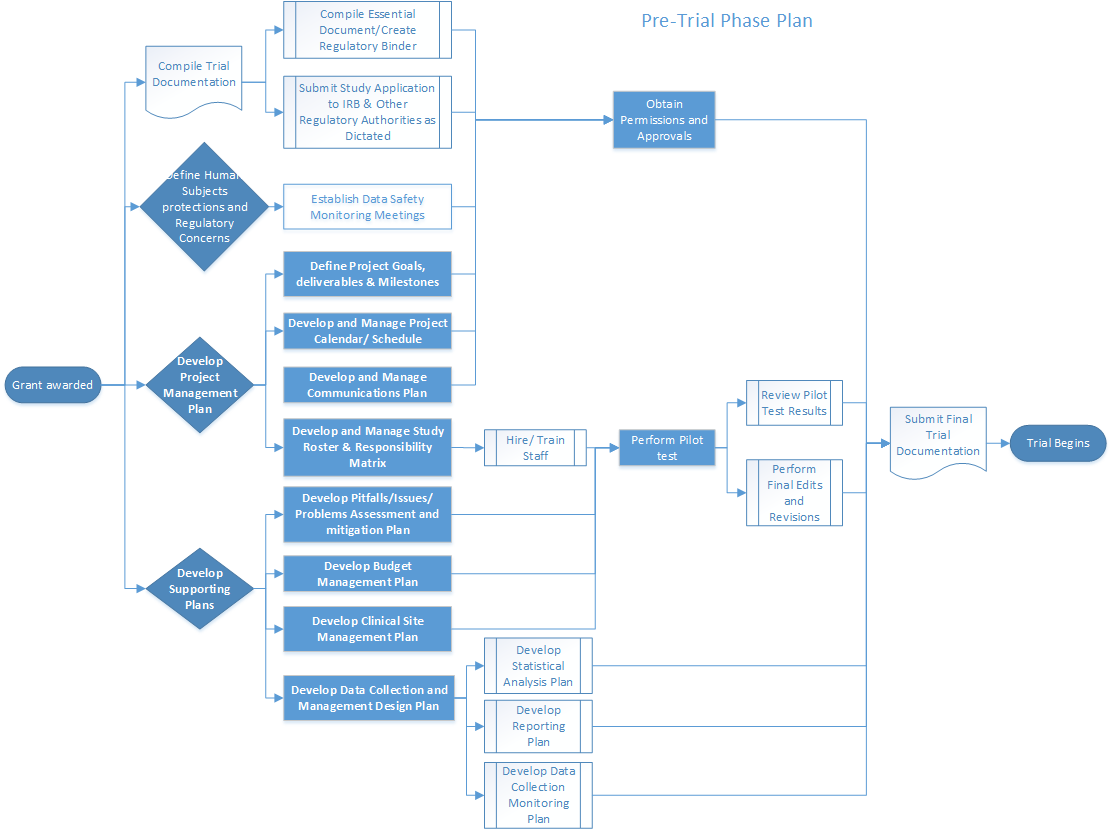
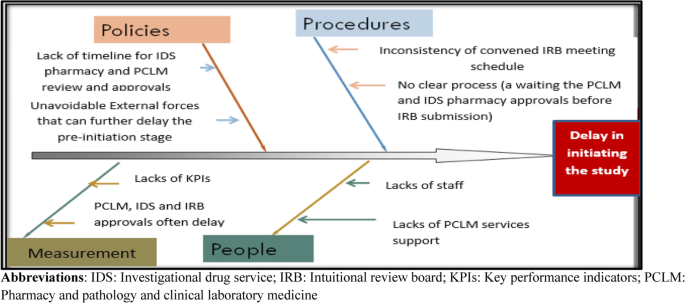
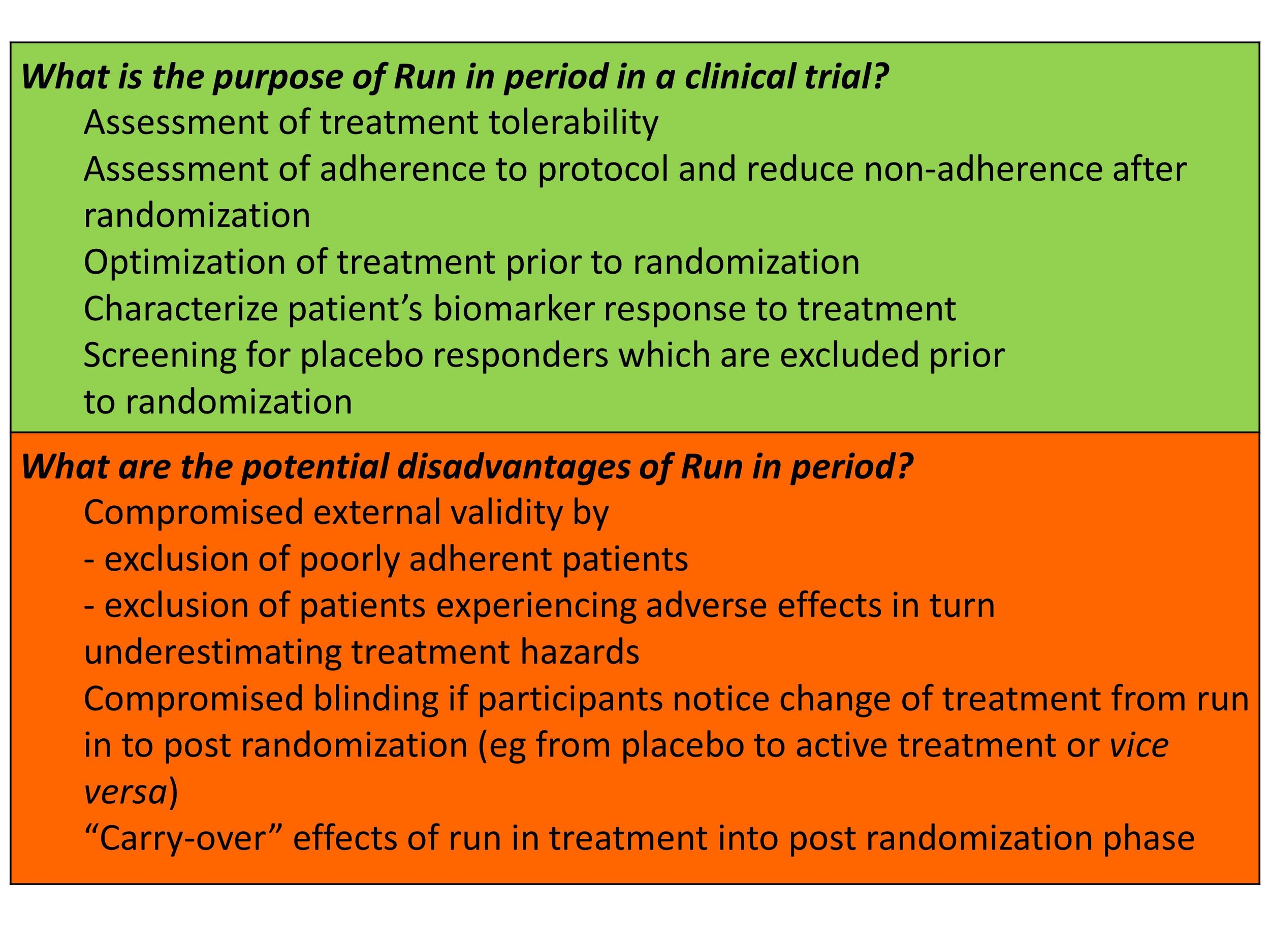
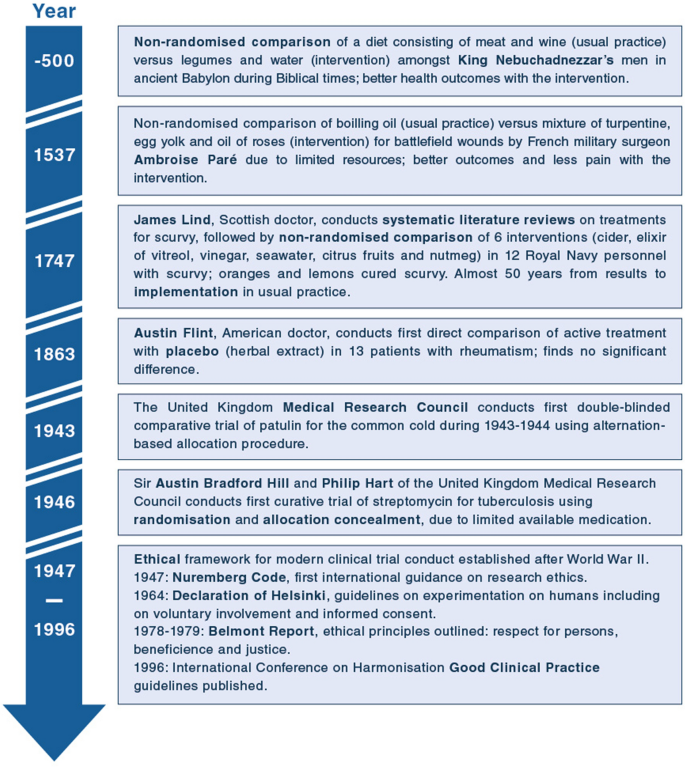
Results from clinical trial simulations evaluating the effect of run-in... | Download Scientific Diagram

Tixagevimab–cilgavimab for treatment of patients hospitalised with COVID-19: a randomised, double-blind, phase 3 trial - The Lancet Respiratory Medicine
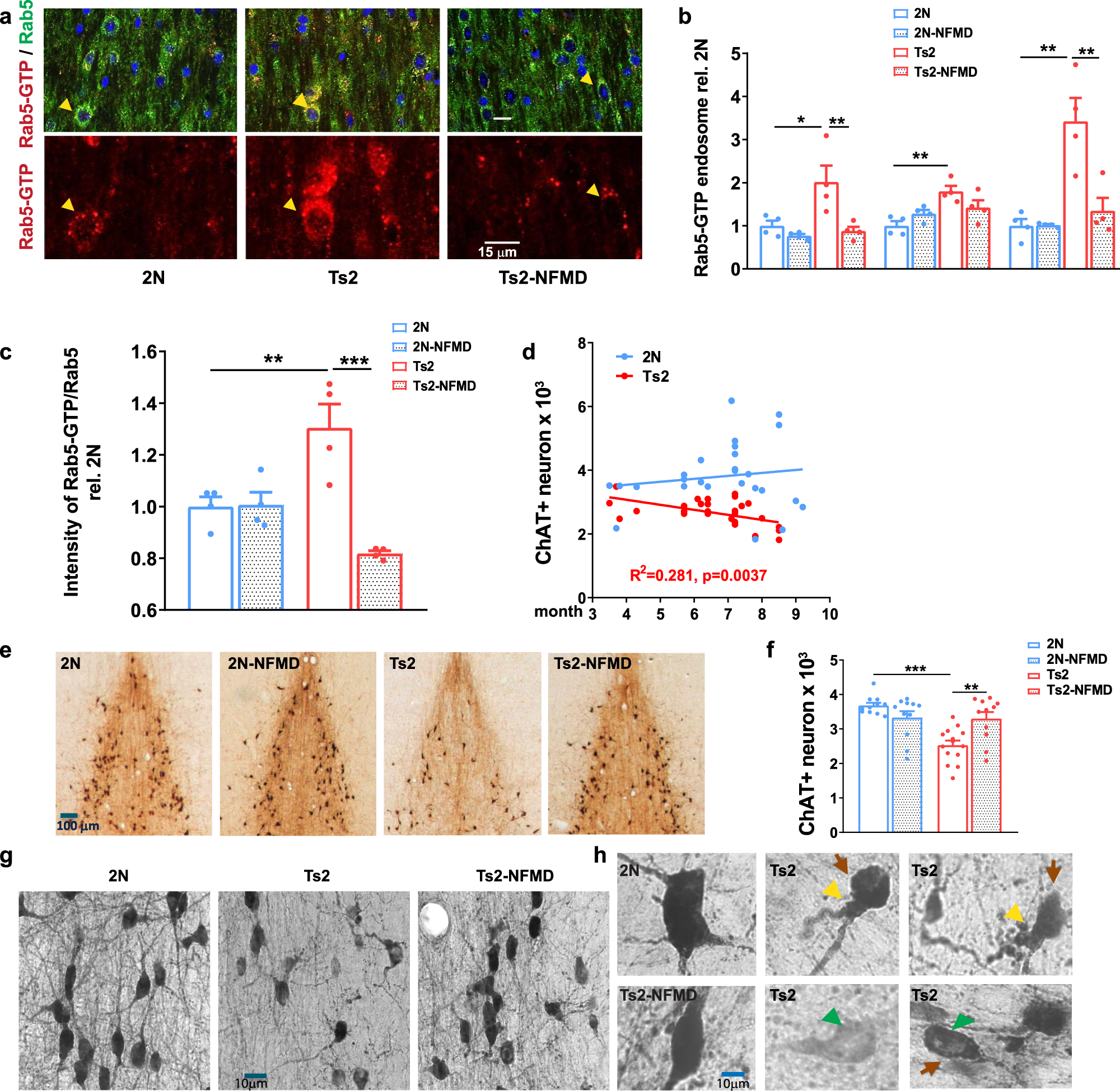
Preclinical and randomized clinical evaluation of the p38α kinase inhibitor neflamapimod for basal forebrain cholinergic degeneration | Nature Communications