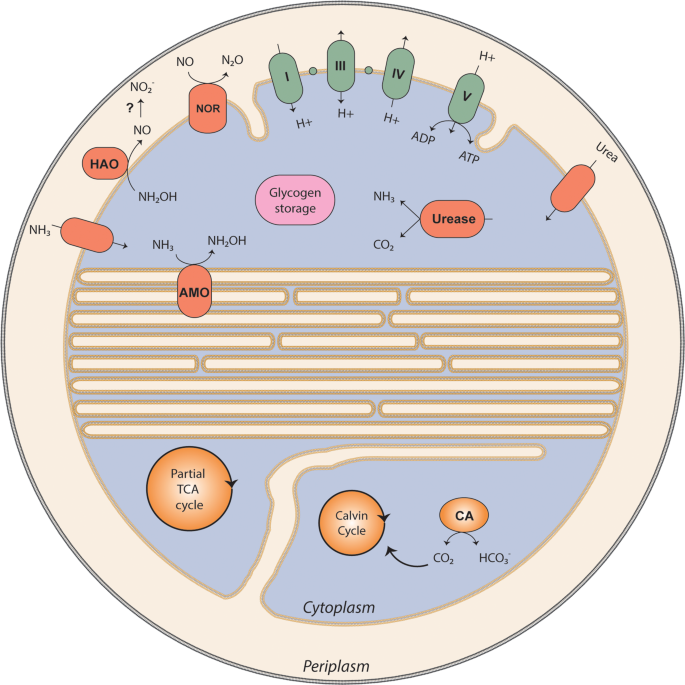
Ammonia oxidation at pH 2.5 by a new gammaproteobacterial ammonia-oxidizing bacterium | The ISME Journal
Buffering capacity β of (a) water, (b) ammonia or ammonium, and (c)... | Download Scientific Diagram

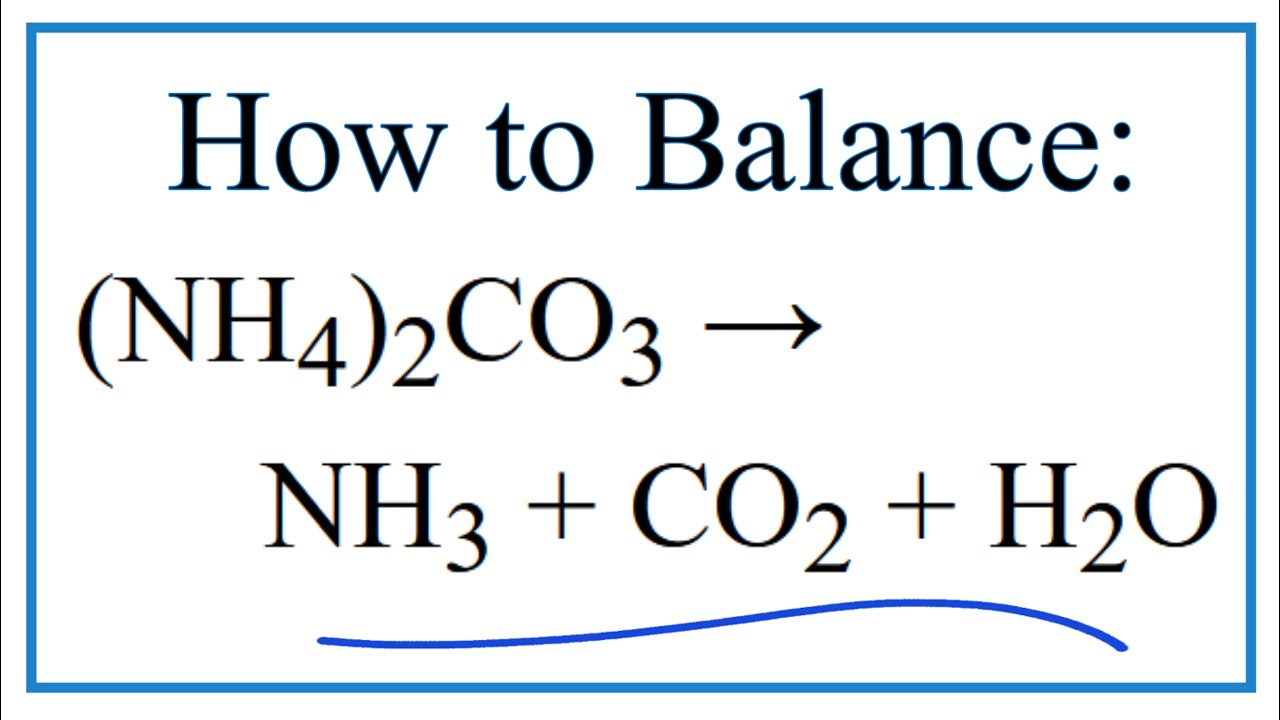
How to Balance (NH4)2CO3 + NaOH = Na2CO3 + NH3 + H2O (Ammonium carbonate + Sodium hydroxide) - YouTube
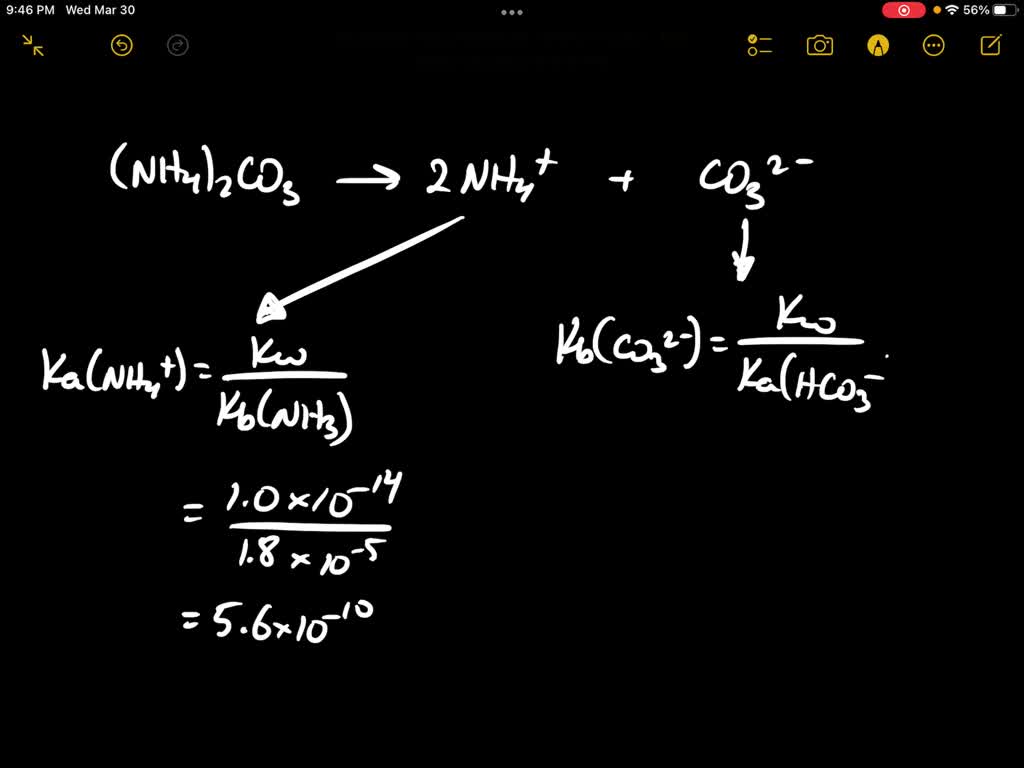
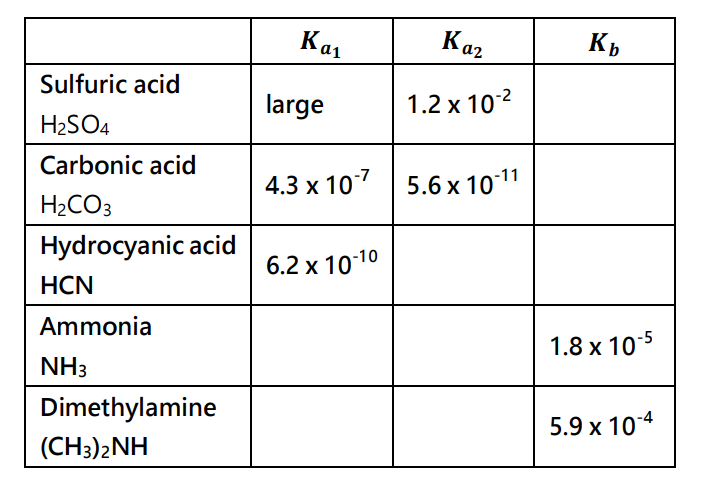
SOLVED: Which of the following options best describes a solution of (NH4)2CO3 at 25 oC? The Kb of ammonia, NH3 is 1.8××10−5. For carbonic acid (H2CO3), Ka1 = 4.5××10−7 and Ka2 =
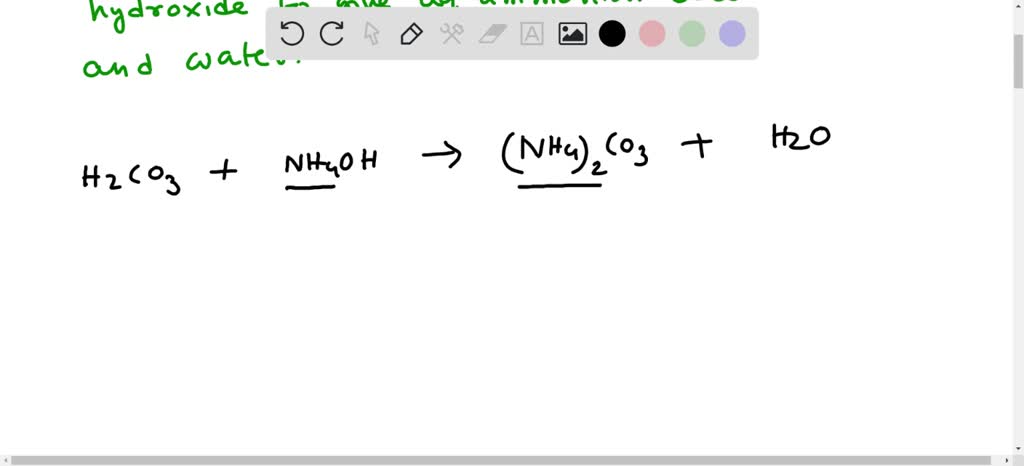
SOLVED: balance the chemical equation carbonic acid reacts with aqueous ammonium hydroxide to give aqueous ammonium carbonate and water

How would ethanoic acid react with ammonia (NH3) and sodium hydroxide (NaOH) respectively? | Homework.Study.com

Which is an Intermediate Species for Photocatalytic Conversion of CO2 by H2O as the Electron Donor: CO2 Molecule, Carbonic Acid, Bicarbonate, or Carbonate Ions? | The Journal of Physical Chemistry C

Ammonia Recovery from Hydrolyzed Human Urine by Forward Osmosis with Acidified Draw Solution | Environmental Science & Technology




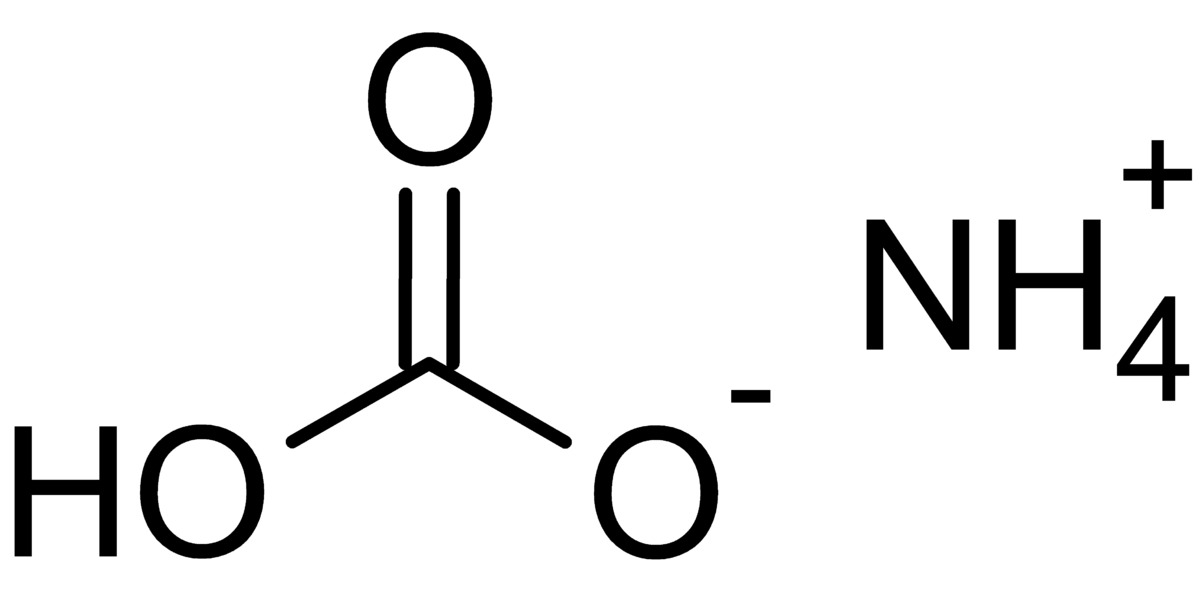


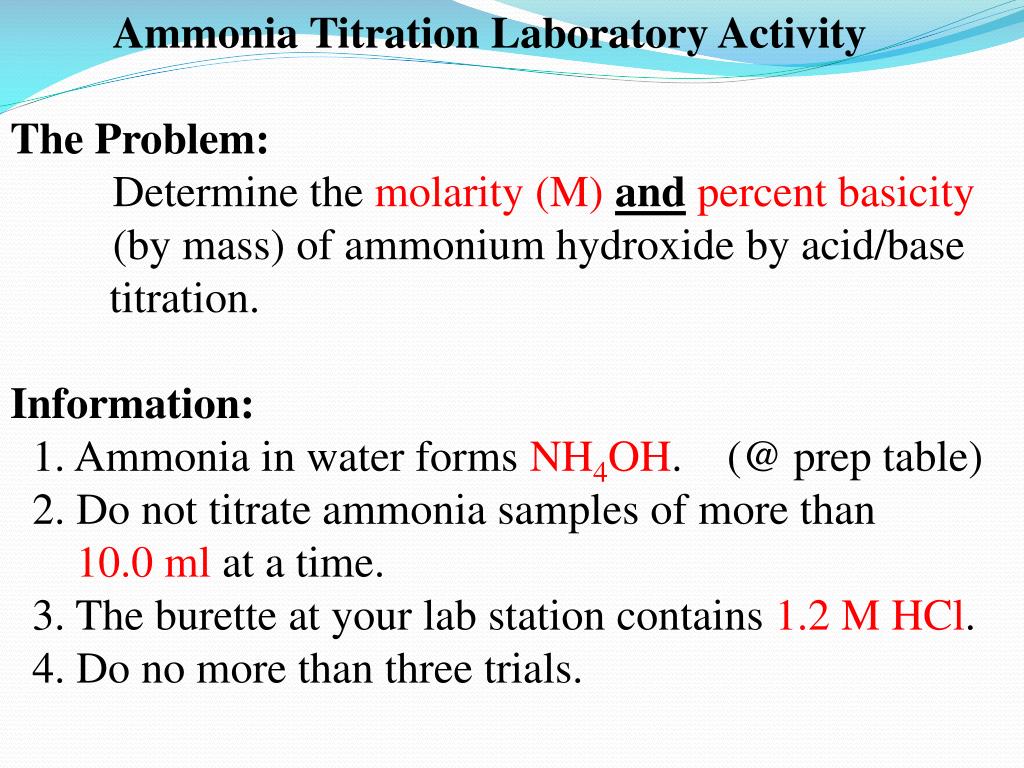


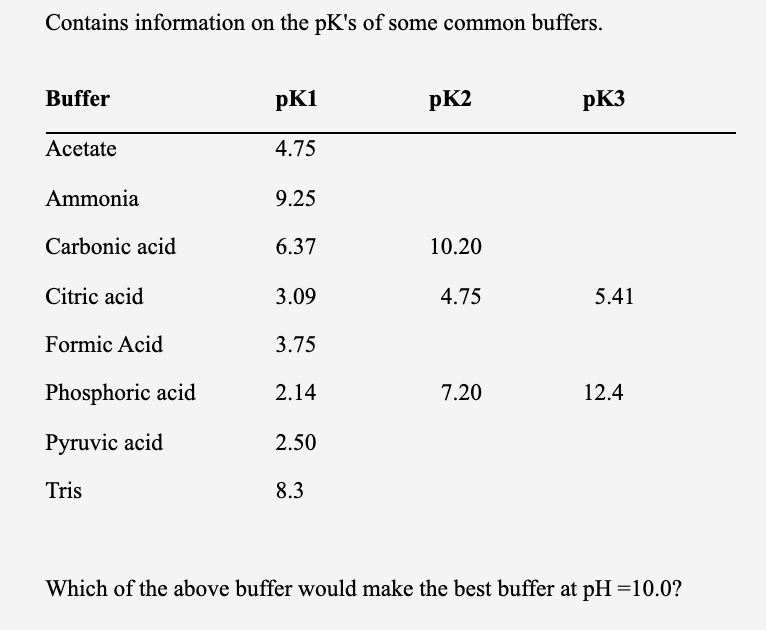
2CO%20+%20H2O%20=%20NH3%20+%20CO2.svg)
