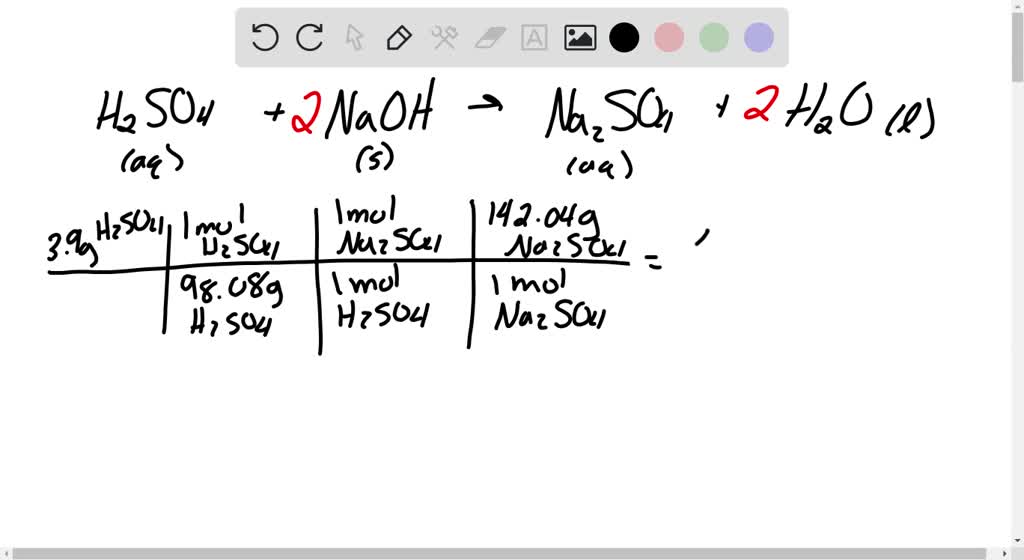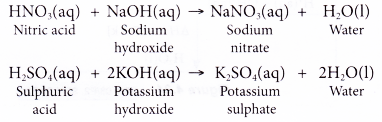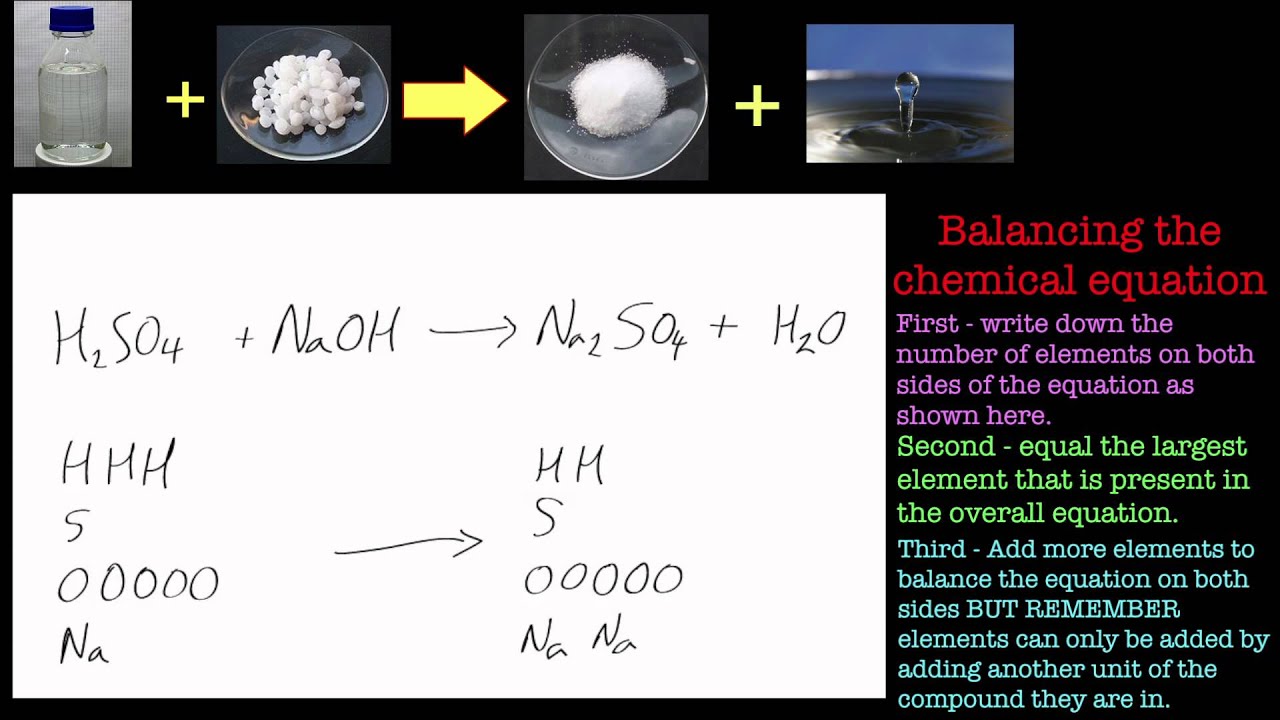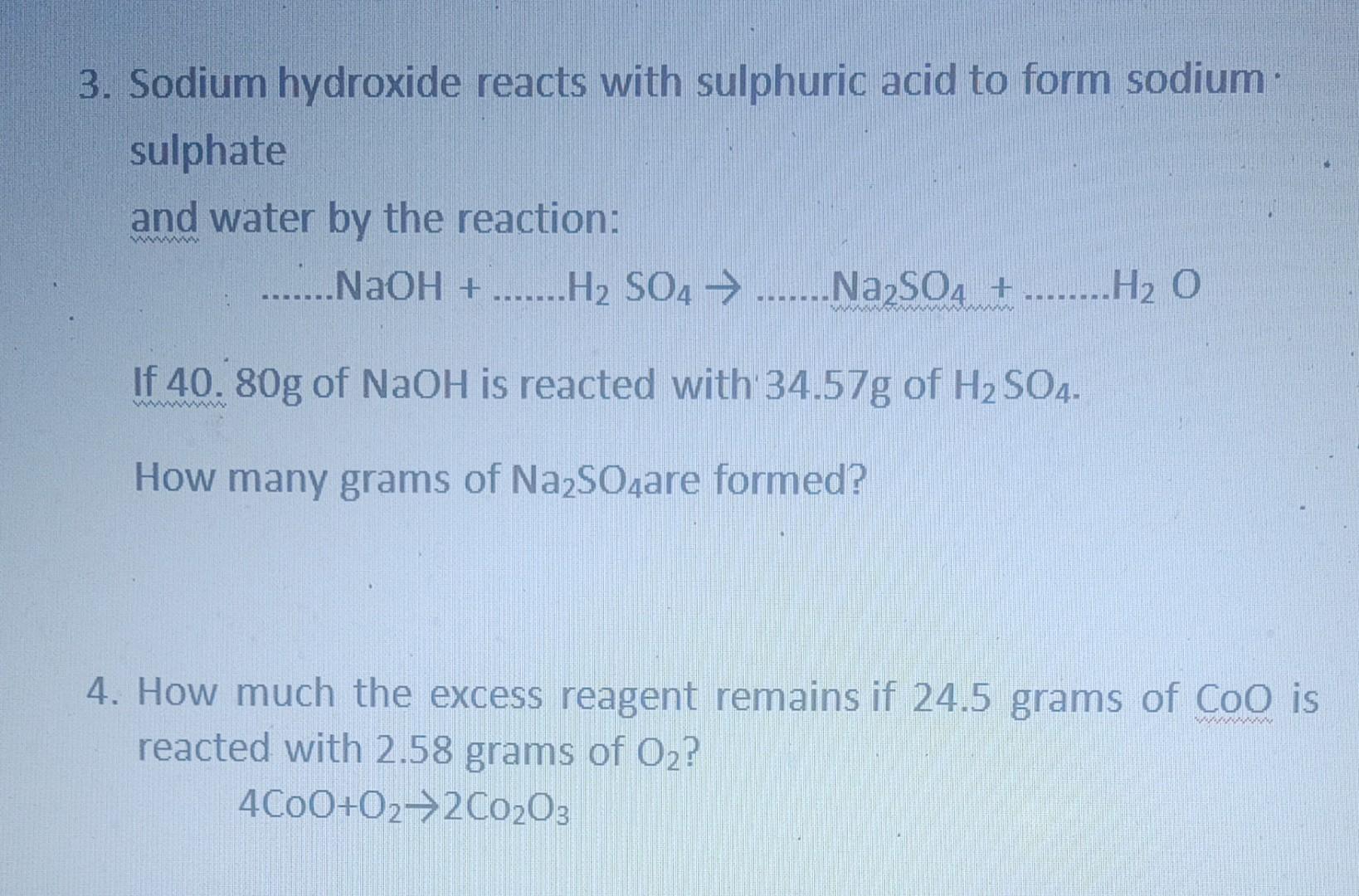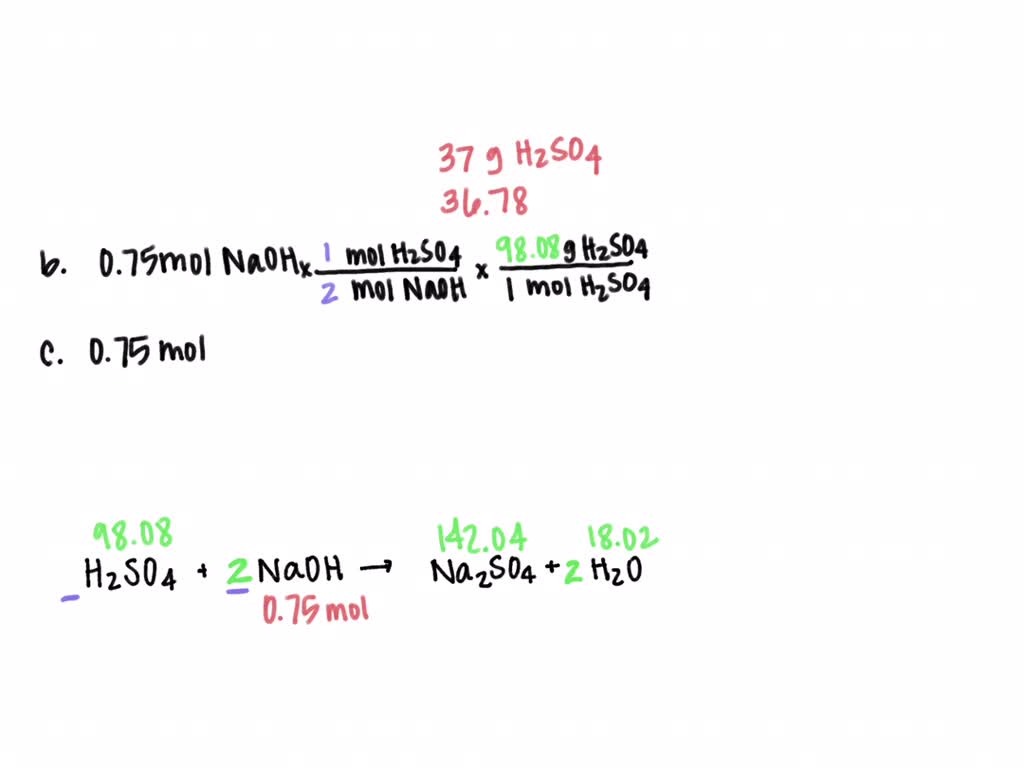
SOLVED: Sulfuric acid reacts with sodium hydroxide according to the following: H2SO4+NaOH⟶Na2SO4+H2O a. Balance the equation for this reaction. b. What mass of H2SO4 would be required to react with 0.75 molNaOH ?
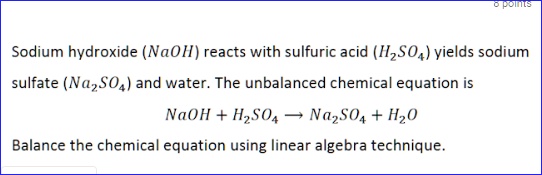
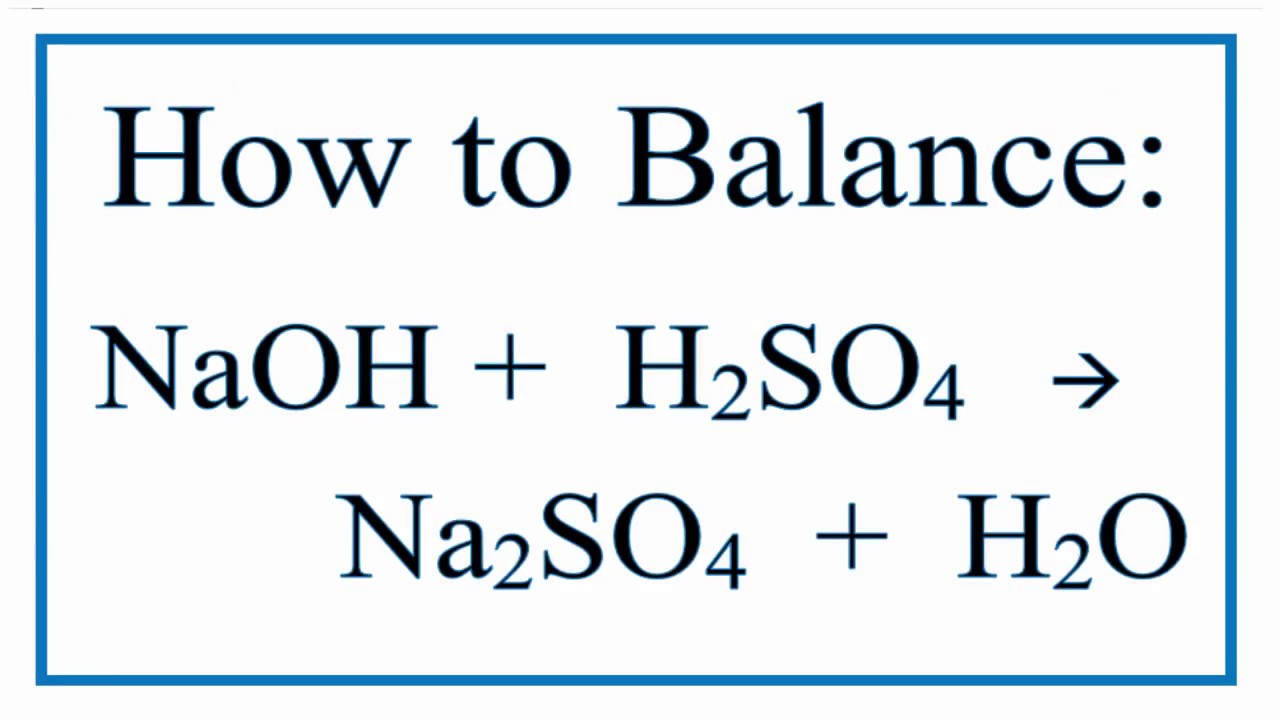
SOLVED: Sodium hydroxide (NaOl) reacts with sulfuric acid (IlzSO4) yields sodium sulfate (Na,SO4) and water. The unbalanced chemical equation is NaOH + HzS04 NazSO4 + Hz0 Balance the chemical equation using linear

Sulfuric Acid LO: Outline uses and reactions involving Sulfuric Acid Starter: What is an acid? - ppt download
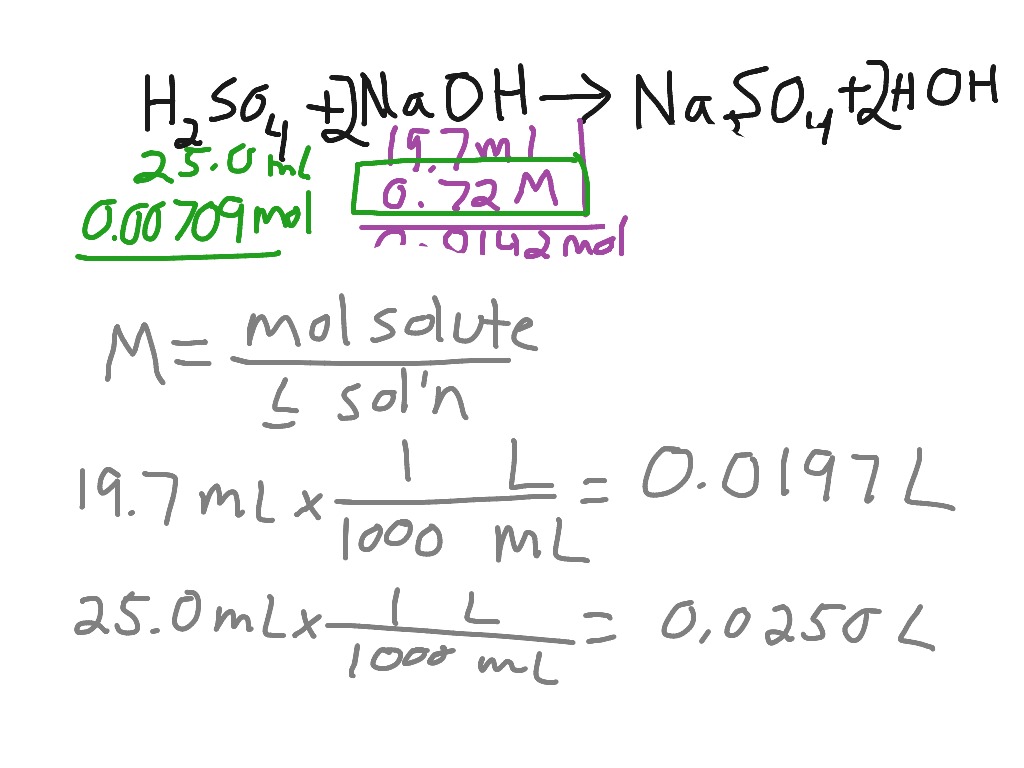
Titration of sulfuric acid with sodium hydroxide | Chemistry, Acids and Bases, Stoichiometry | ShowMe
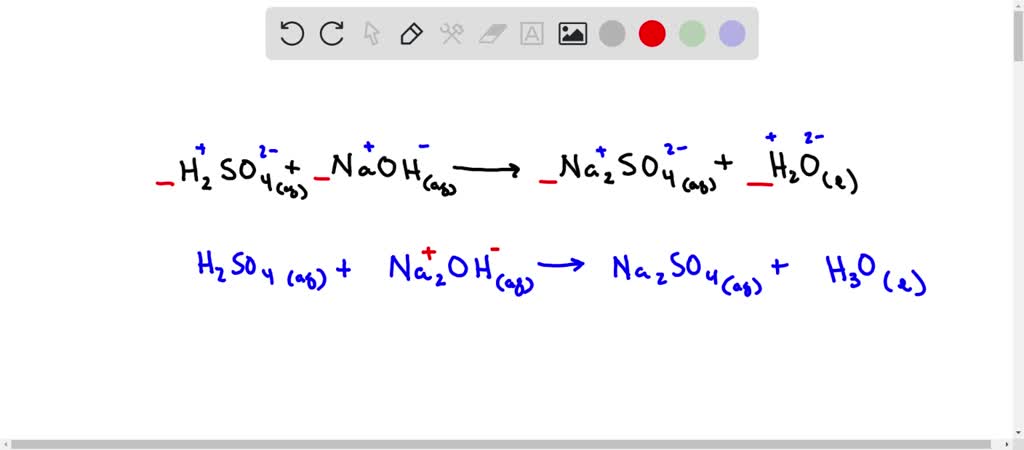
SOLVED: Sulfuric acid, H2SO4, can be neutralized by sodium hydroxide, NaOH. The unbalanced equation is:H2SO4(aq) + NaOH(aq) → Na2SO4(aq) + H2O(l)A student who was asked to balance the reaction wrote the following:H2SO4(aq) +

Question Video: Calculating the Volume of Sulfuric Acid That Completely Neutralizes a Given Volume and Concentration of Sodium Hydroxide | Nagwa

Sulphuric acid reacts with sodium hydroxide as follows H(2)SO(4)+2NaOHrarrNa(2)SO(4)+2H(2)O when 1L of 0.1M sulphuric acid solution is allowed to react with 1L of 0.1M sodium hydroxide solution, the amount of sodium solphate

Sulphuric acid reacts with sodium hydroxide as follows: H2SO4 + 2NaOH→ Na2SO4 + 2H2O When 1 L of 0.1 M sulphuric acid solution is allowed to react with 1 L of 0.1

OneClass: Aqueous sulfuric acid H2SO4 will react with solid sodium hydroxide NaOH to produce aqueous ...

2.46 g of sodium hydroxide (molar mass = 40) are dissolved in water and the solution is made to 100 cm ^3 in a volumetric flask. Calculate the molarity (in mol/L) of the solution.
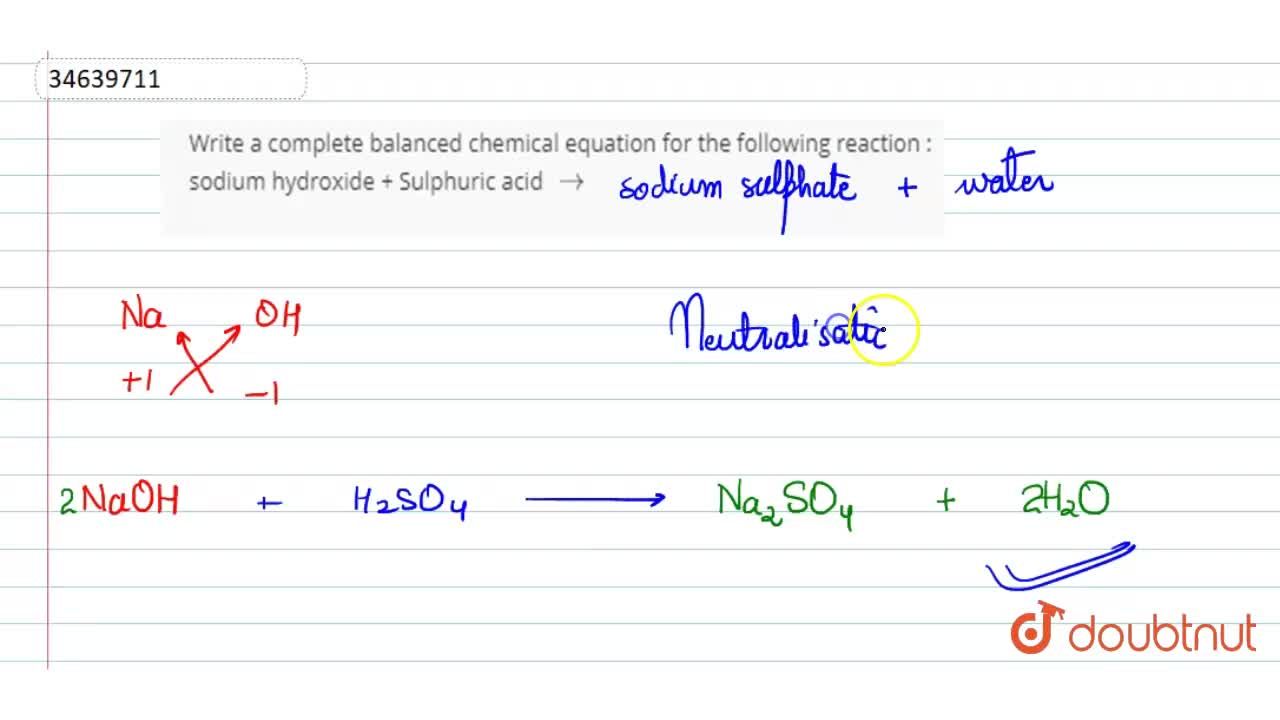
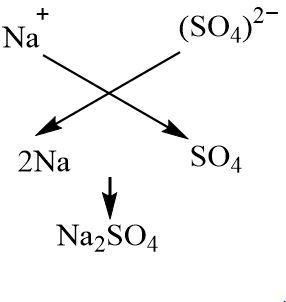
Write a complete balanced chemical equation for the following reaction : sodium hydroxide + Sulphuric acid to