
The `pK_(a)` of acetic acid and `pK_(b)` of ammonium hydroxide are `4.76` and `4.75` respectivel... - YouTube

What are the concentrations of acetic acid ( p K a = 4.76 ) and acetate in a buffer solution of 0.10 M at pH 4.6? (acetic acid) = _____ M (acetate)= _____ M | Homework.Study.com

OneClass: What concentrations of acetic acid (pKa = 4.76) and acetate would be required to prepare a ...
Acid Dissociation Constant (pKa) of Common Monoethylene Glycol (MEG) Regeneration Organic Acids and Methyldiethanolamine at Vary

Experimental pKa values and structures of the conformers of acetic,... | Download Scientific Diagram

pKa value of acetic acid is 4.75. If the buffer solutions contains 0.125 M acetic acid and 0,25 M sodium acetate the pH of buffer solution is :
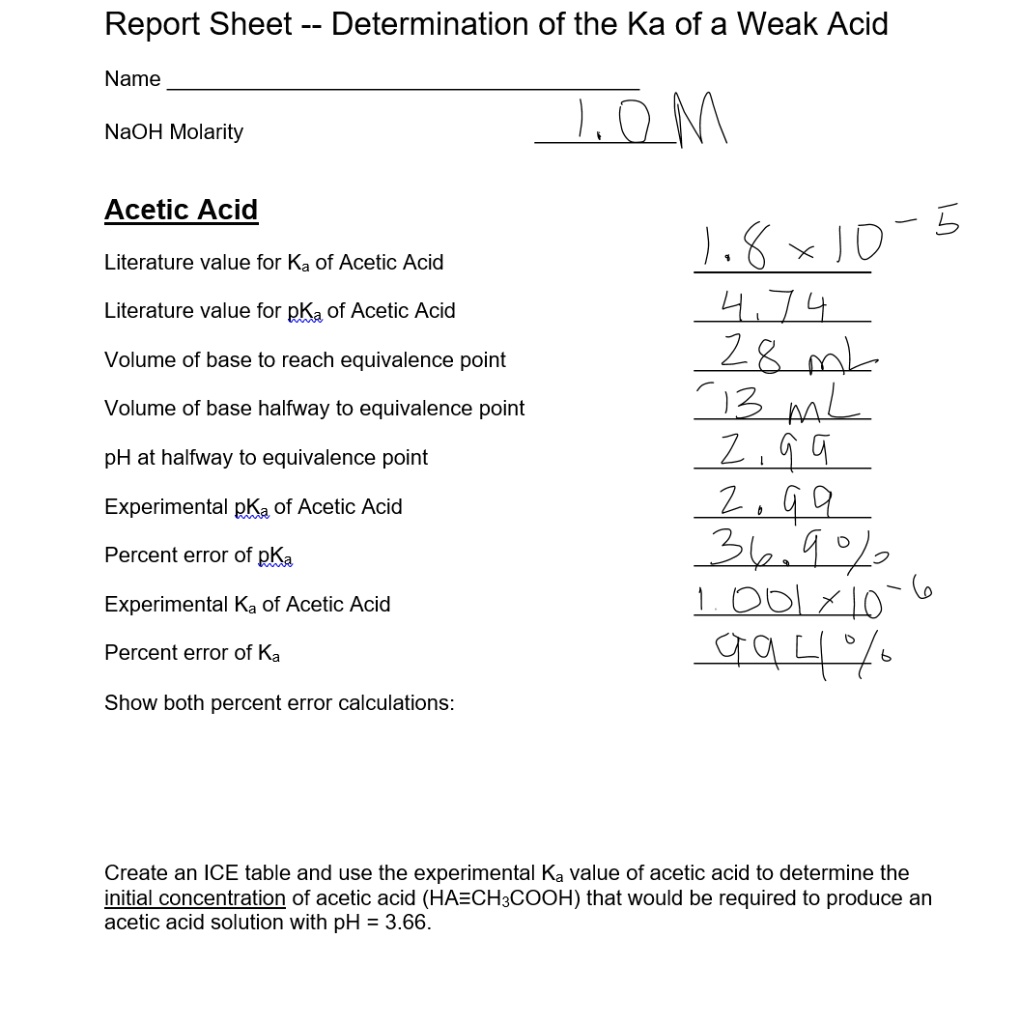
SOLVED: Report Sheet Determination of the Ka of a Weak Acid Name NaOH Molarity IM Acetic Acid Literature value for Ka of Acetic Acid Literature value for pKa of Acetic Acid LkxD -

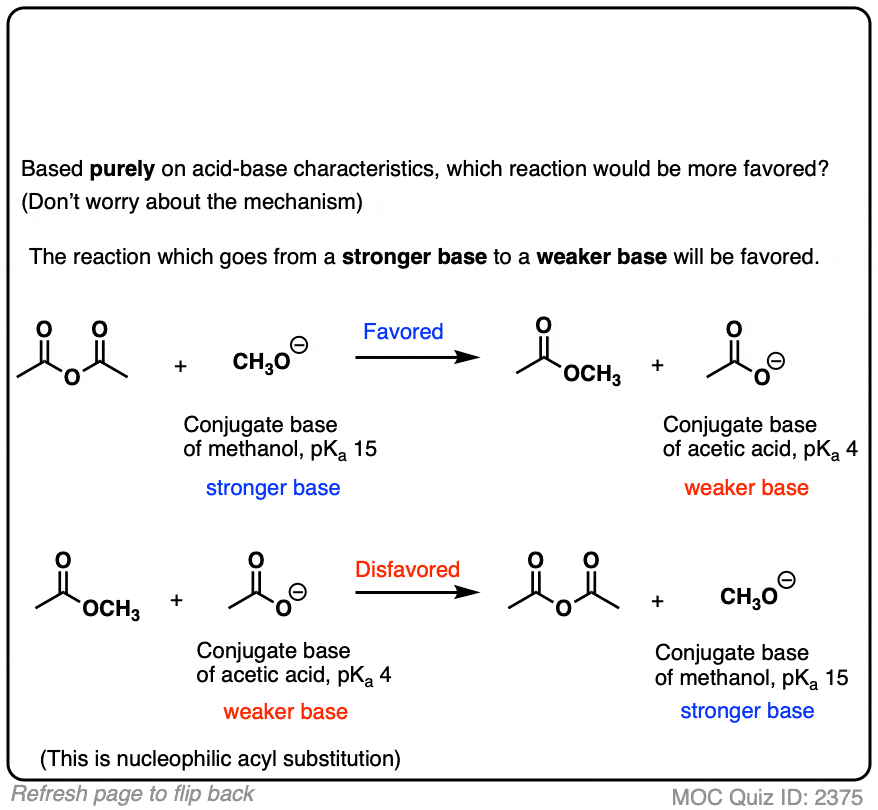
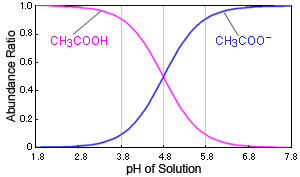
3.3: pKa of Organic Acids and Application of pKa to Predict Acid-Base Reaction Outcome - Chemistry LibreTexts

Acid Dissociation Constant (pKa) of Common Monoethylene Glycol (MEG) Regeneration Organic Acids and Methyldiethanolamine at Varying MEG Concentration, Temperature, and Ionic Strength | Journal of Chemical & Engineering Data

OneClass: What are the concentrations of acetic acid (pKa = 4.76) and acetate in a buffer solution of...
The pKa value of acetic acid is 4.7447 at 25°C. How would you obtain a buffer of acetic acid and sodium acetate with pH = 4? - Sarthaks eConnect | Largest Online Education Community

SOLVED: Calculate the ratio of acetic acid to acetate concentration needed to prepare a buffer with a pH of 5.80. Acetic acid pka = 4.70.
Acetic acid has a pKa of 4.8, ethanol has a pKa of 16.0. What are the major species present, when acetic acid and ethanol are added to water and then the pH




:max_bytes(150000):strip_icc()/what-is-pka-in-chemistry-605521_FINAL2-9fdfc39e9aa34caa96d6e74a2c687707.png)


