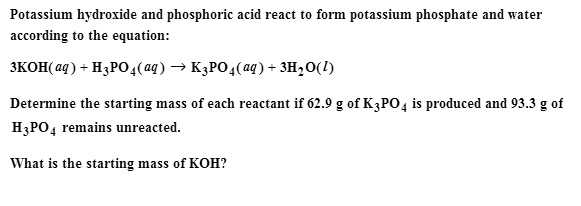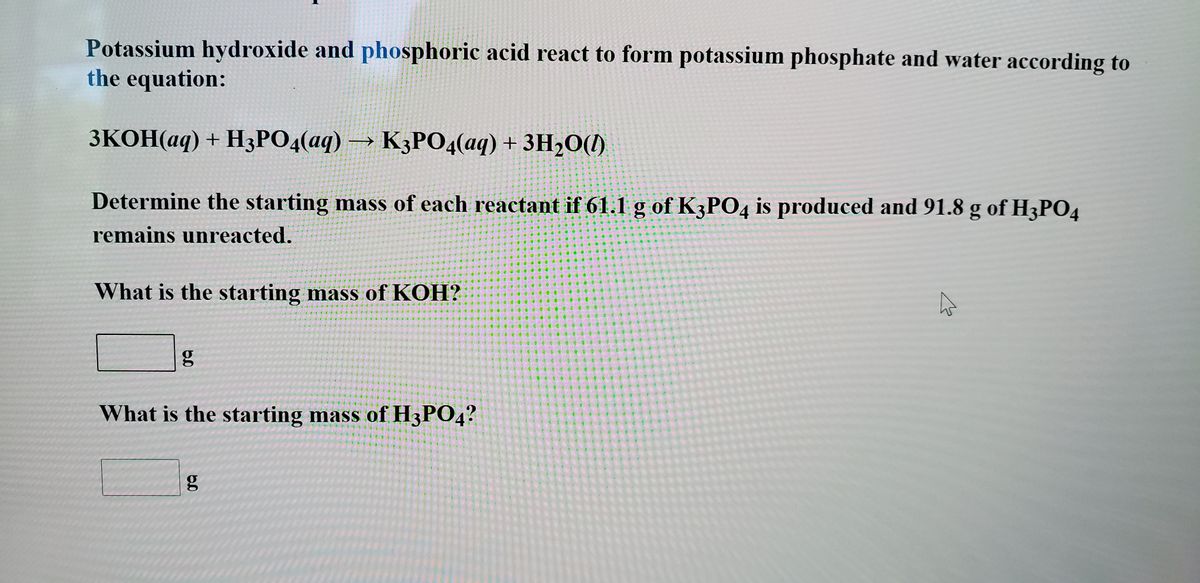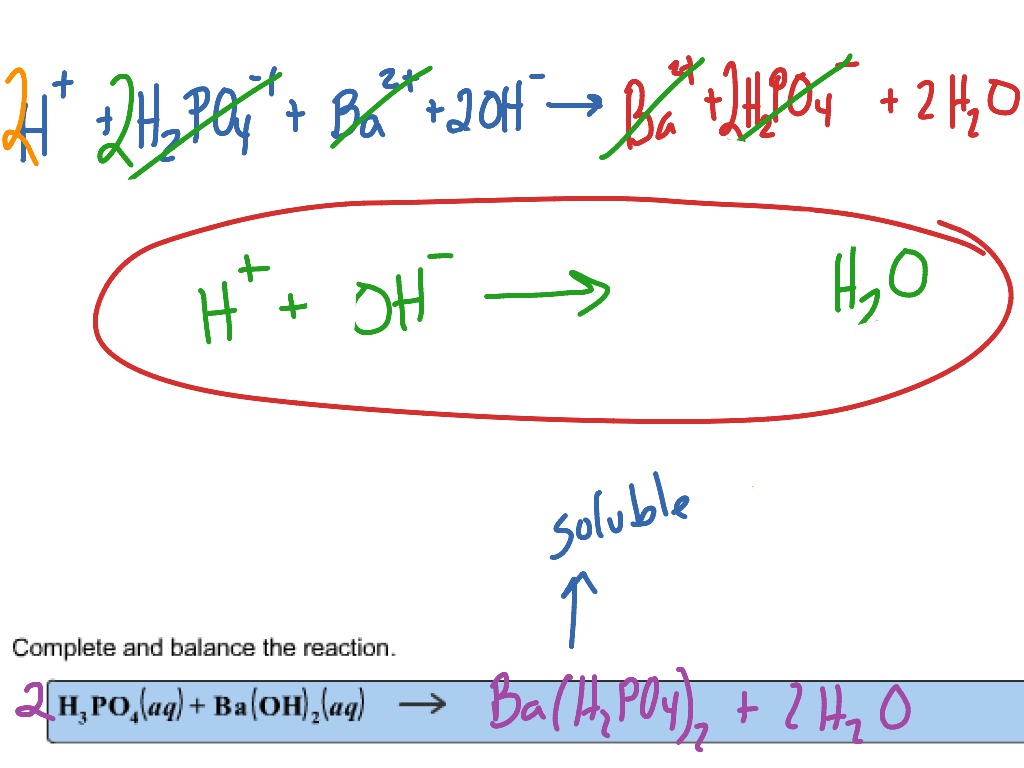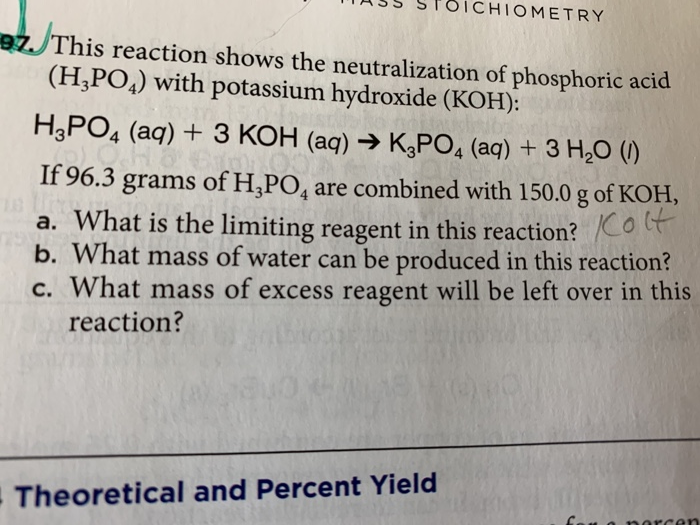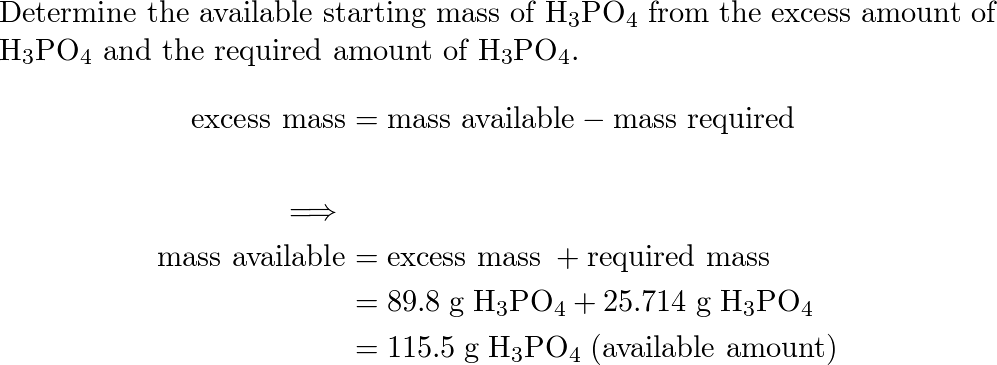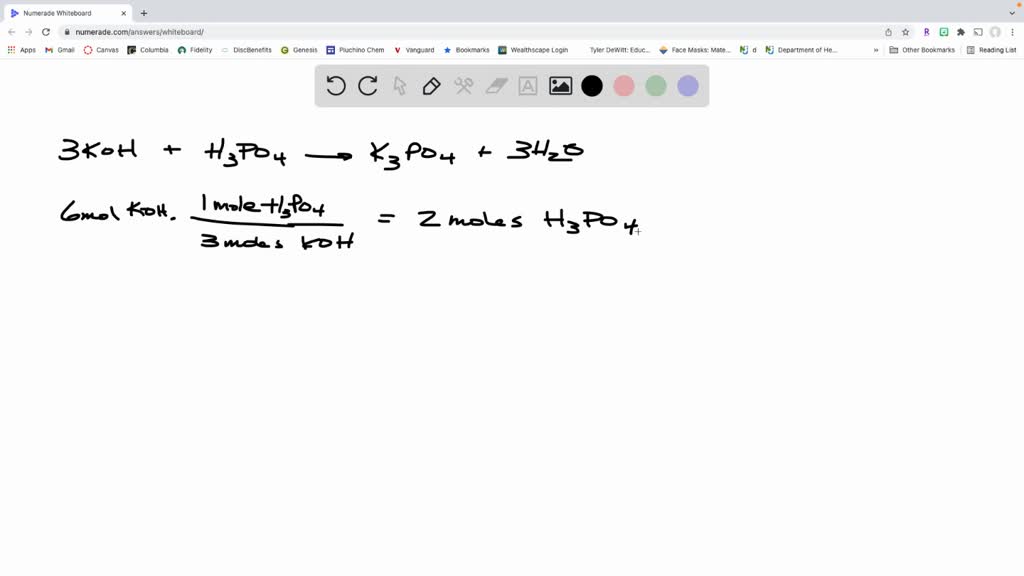
SOLVED: When potassium hydroxide reacts with phosphoric acid, potassium phosphate and water are produced. The balanced equation for this reaction is: 3KOH(aq) + H3PO4 (aq) -> K3PO4(aq) + 3H2O(l) If 6 moles
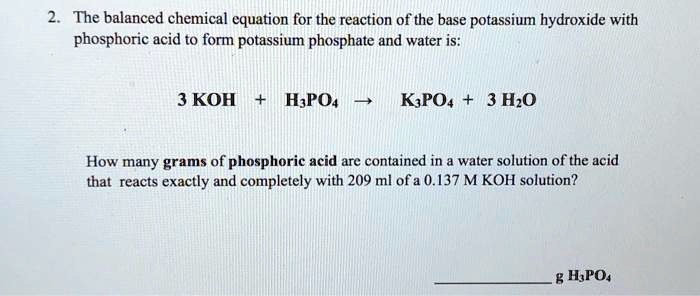
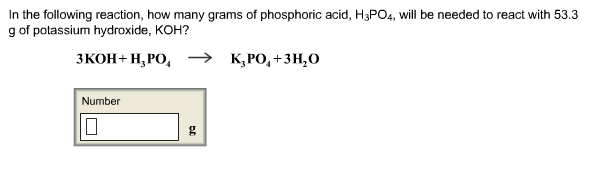
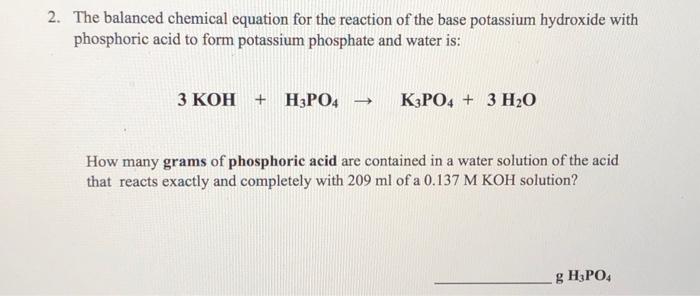
SOLVED: The balanced chemical equation for the reaction of the base potassium hydroxide with phosphoric acid to form potassium phosphate and water is: 3 KOH HPO4 KaPOs 3 HzO How many grams

Outline of AC preparation from RH. In figure, KOH, ZnCl2, H3PO4, and N... | Download Scientific Diagram
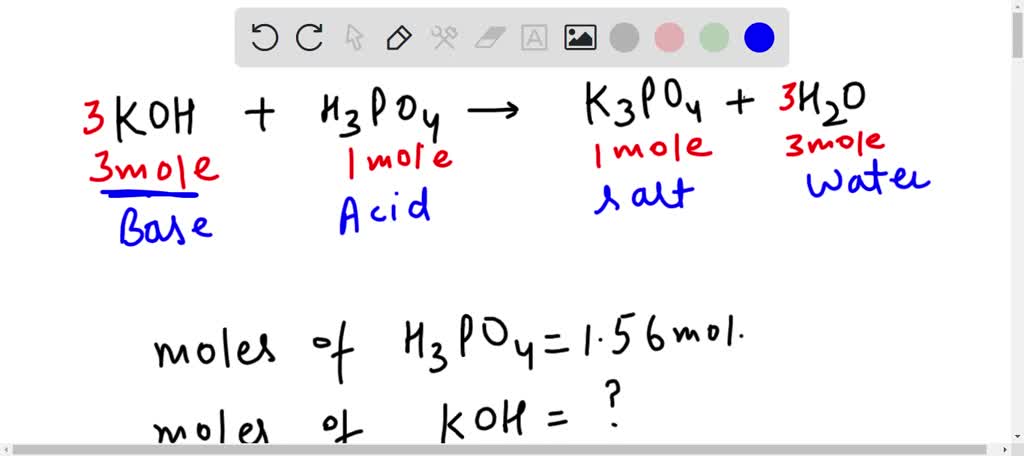
SOLVED: How many moles of potassium hydroxide (KOH) are needed to completely neutralize 1.56 mol phosphoric acid (H3PO4)?

Ch19.1 – Acids and Bases Acids - corrosive, taste sour, put electrolytes in soln, react with metals Ex1) Single displacement reaction: H 2 SO 4(aq) + Zn. - ppt download
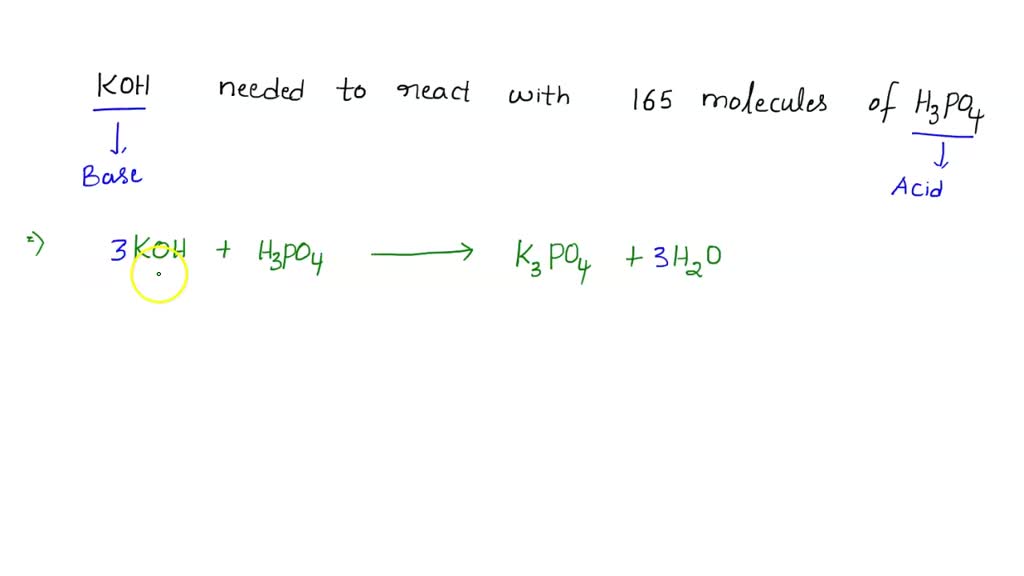
SOLVED: 'How many units of potassium hydroxide are needed to react with 165 molecules of phosphoric acid? BKOHq) HzPO4aq) KzPO4aq) 3HzQ()'