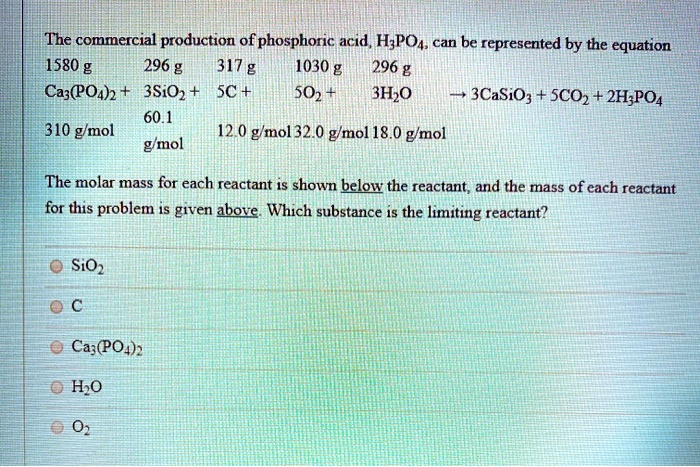
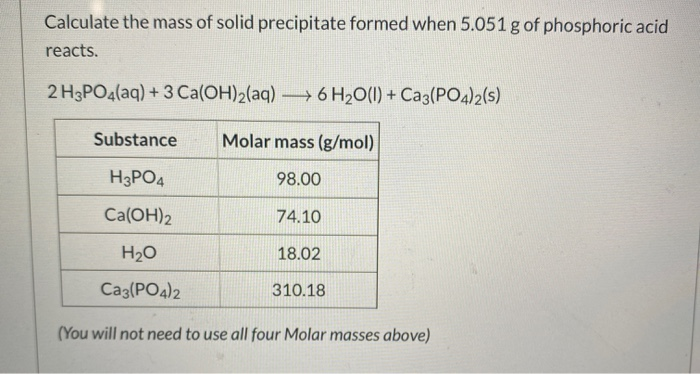
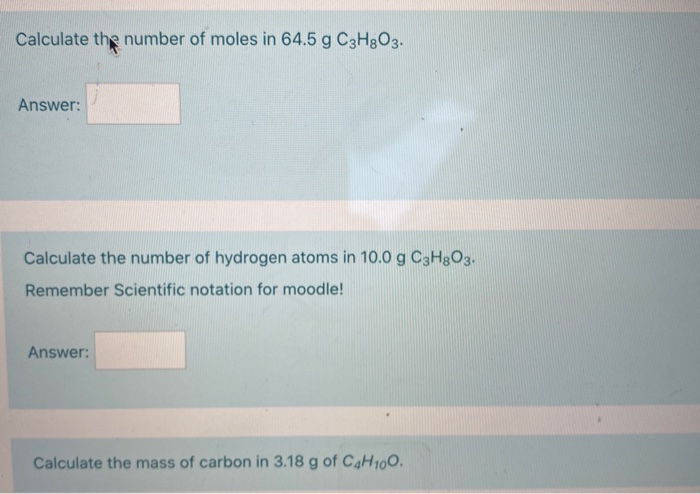
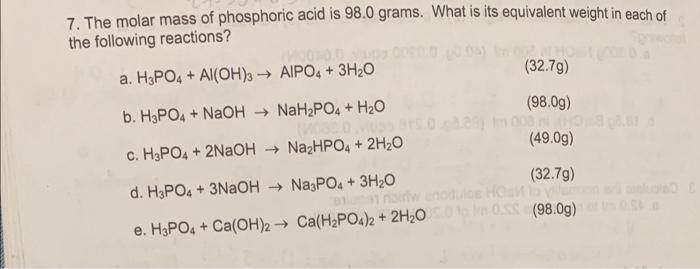The molecular weight of phosphoric acid, H 3 PO 4 , is 97.99 * g/(mol) What is the mass, in grams, of - Brainly.com

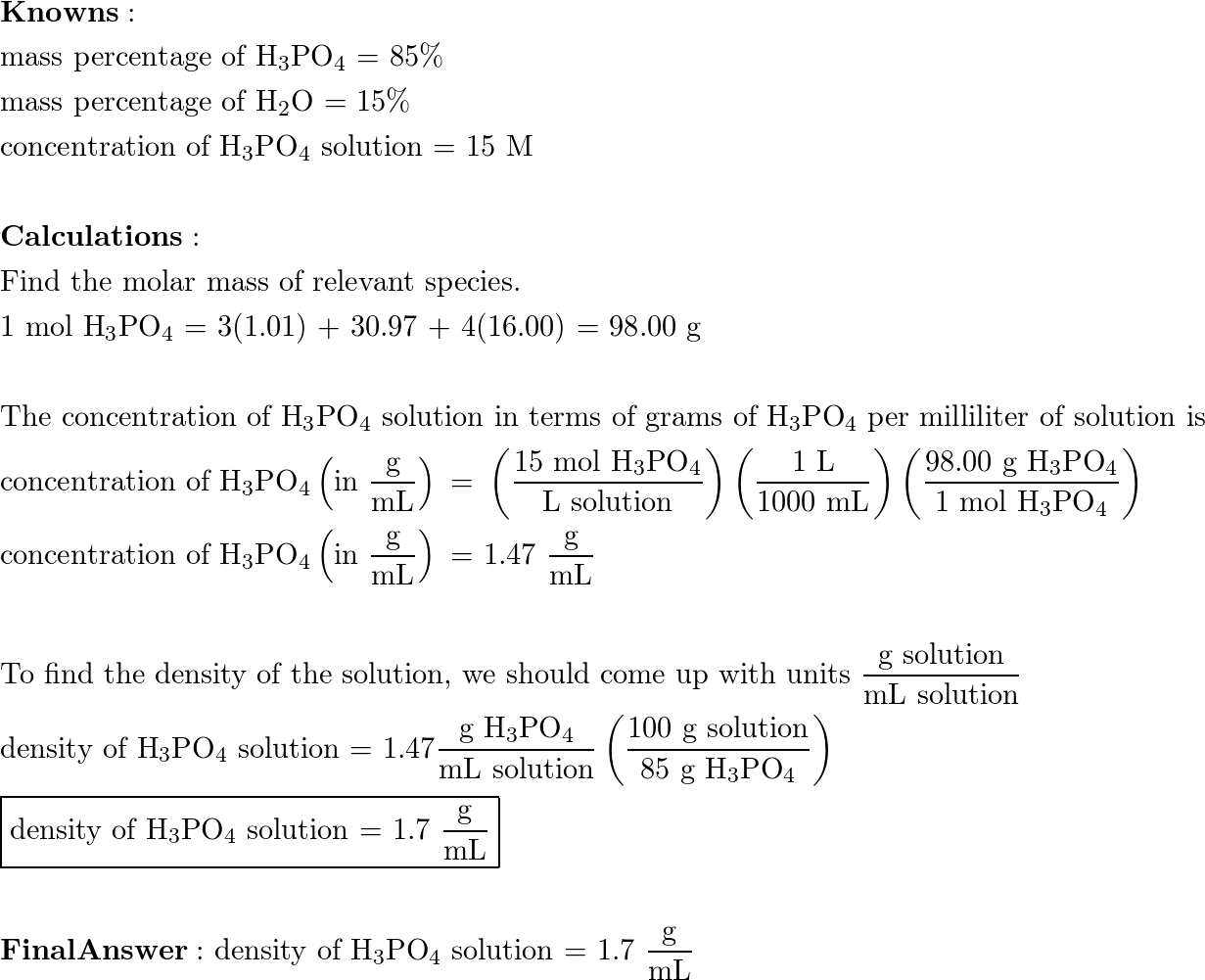
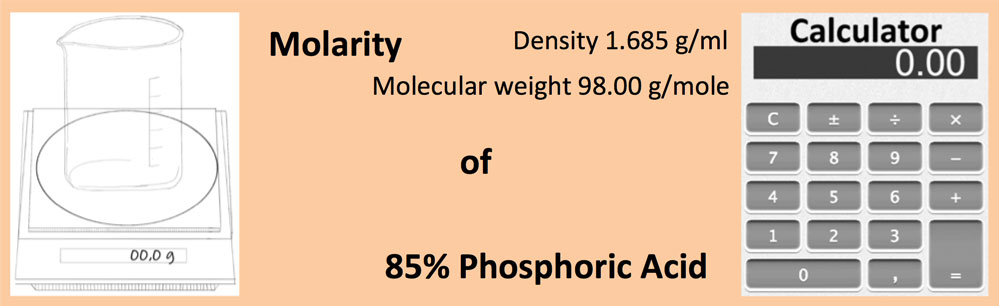
SOLVED: Concentrated phosphoric acid is 90.% H3PO4 by mass and the remaining mass is water: The molarity of H3PO4 in 90% H3PO4 is 12.2 Mat room temperature: What is the density of
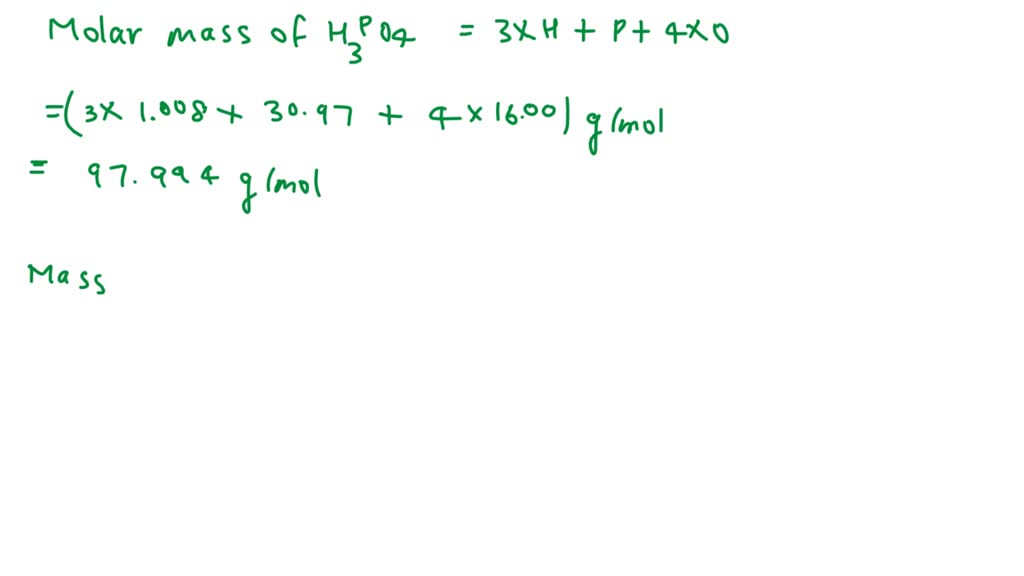
SOLVED: What is the percent of phosphorus by mass in phosphoric acid, H3PO4? Use 1.008 g/mol for the molar mass of hydrogen, 16.00 g/mol for the molar mass of oxygen, and 30.97
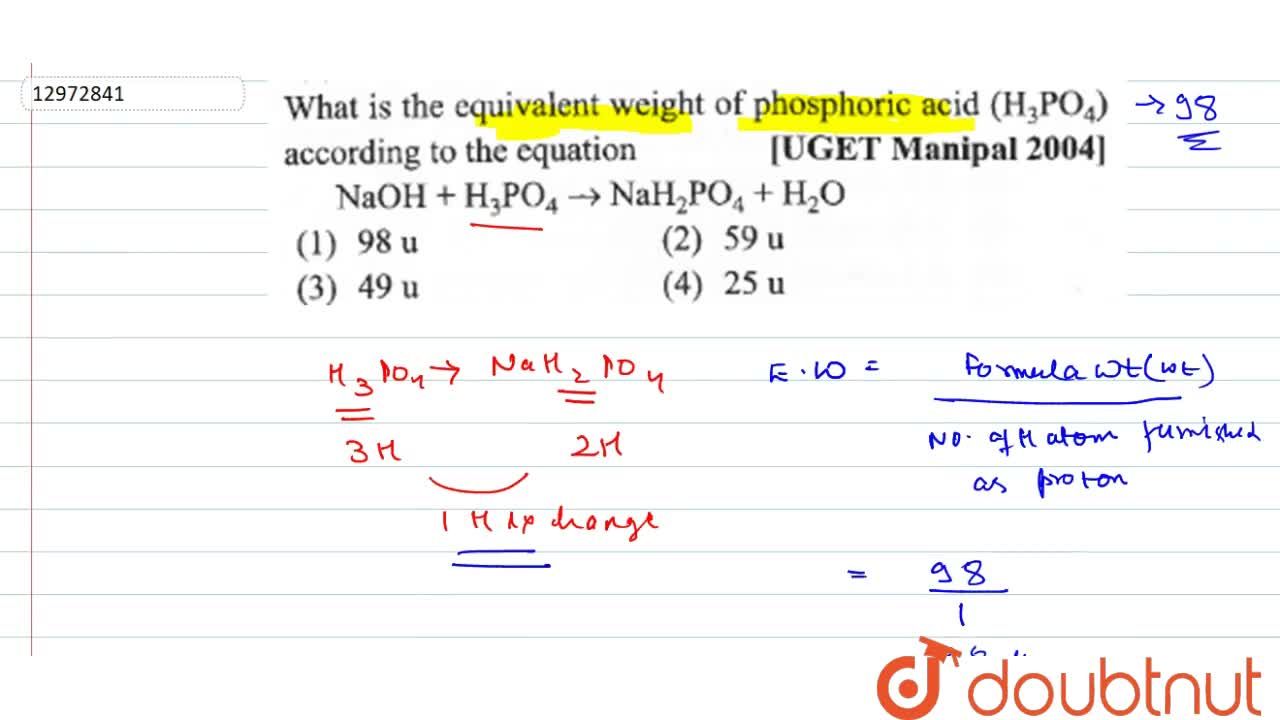
What is the equivalent weight of phosphoric acid (H(3) PO(4)) according to the equation NaOH + H(3) PO(4) rarr NaH(2) PO(4) + H(2)O
![Orthophosphoric Acid (H3PO4) [Phosphoric Acid] - Structure, Formula, Synthesis, Properties, Uses and FAQs of Orthophosphoric acid. Orthophosphoric Acid (H3PO4) [Phosphoric Acid] - Structure, Formula, Synthesis, Properties, Uses and FAQs of Orthophosphoric acid.](https://cdn1.byjus.com/wp-content/uploads/2018/11/phosphoric-acid-structure.png)
Orthophosphoric Acid (H3PO4) [Phosphoric Acid] - Structure, Formula, Synthesis, Properties, Uses and FAQs of Orthophosphoric acid.
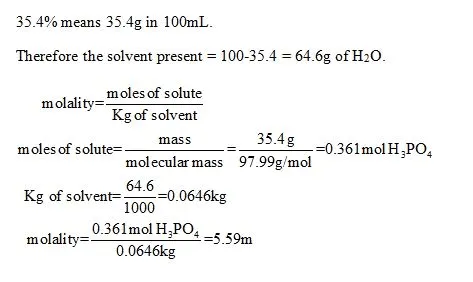
OneClass: Calculate molality of a 35.4% (by mass) aqueous solution of phosphoric acid (H3PO4). The mo...

The equivalent weight of phosphoric acid `(H_(3)PO_(4))` in the reaction `NaOH+H_(3)PO_(4) rarr NaH_ - YouTube