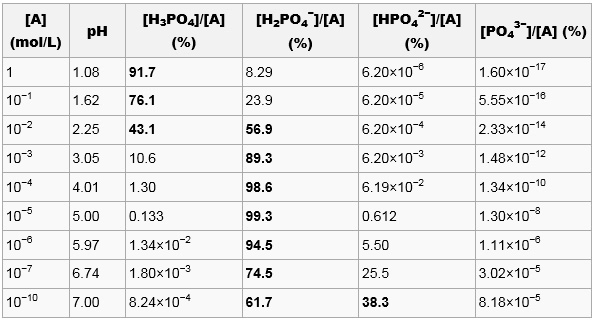
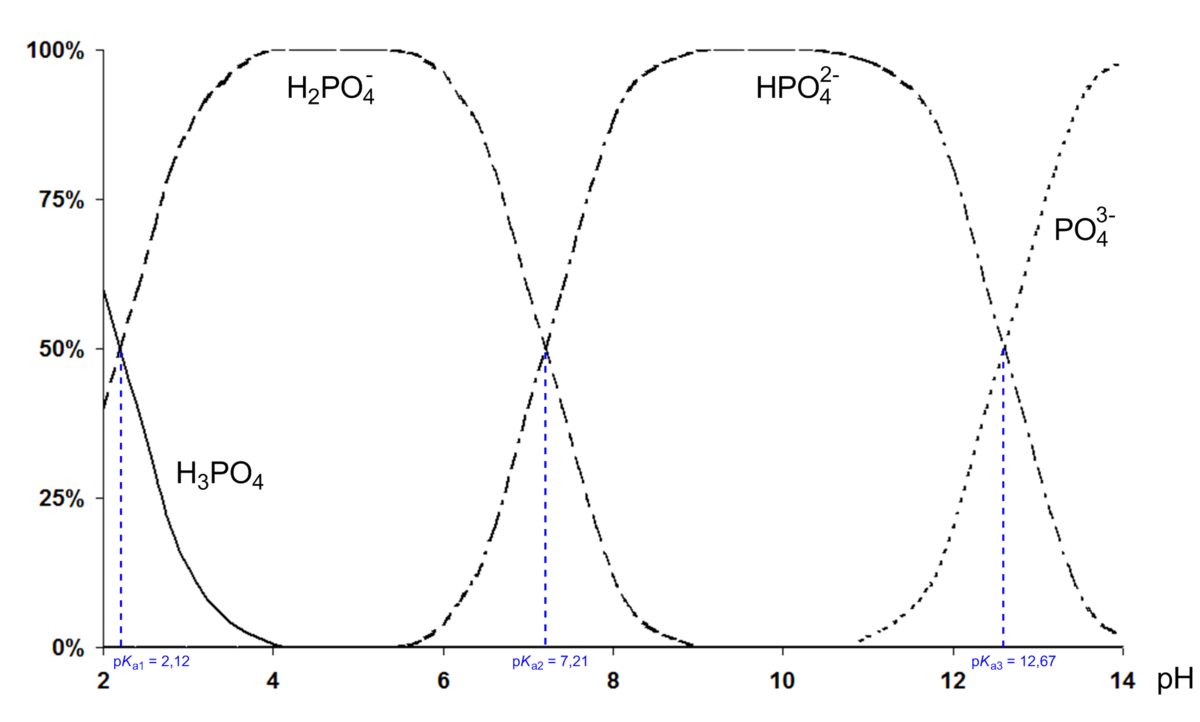
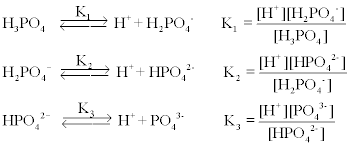OneClass: What is the concentration of phosphate ions in a 2.5 M solution ofphosphoric acid?(The pH o...

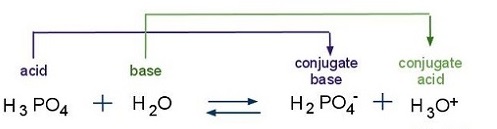
First ionization of phosphoric acid is: H3PO4 H3PO4^- + H^+ pKa1 = 2.21 The dissociation constant of conjugate base of H3PO4 will be:

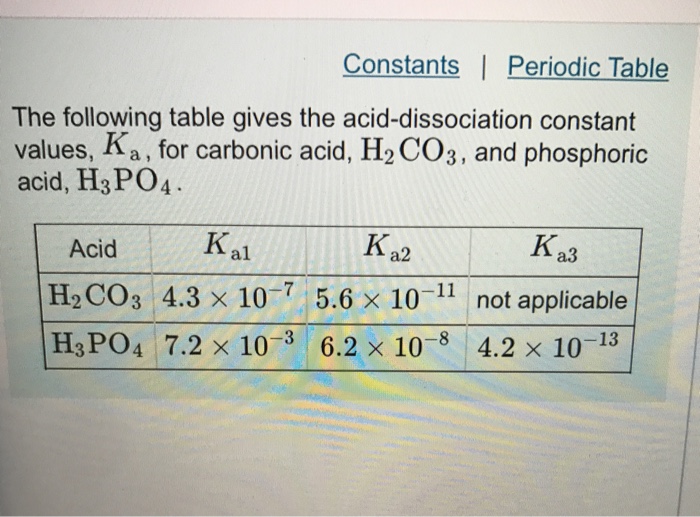
Explain why there are different equilibrium constants, Ka1, Ka2, and Ka3 for phosphoric acid. | Homework.Study.com
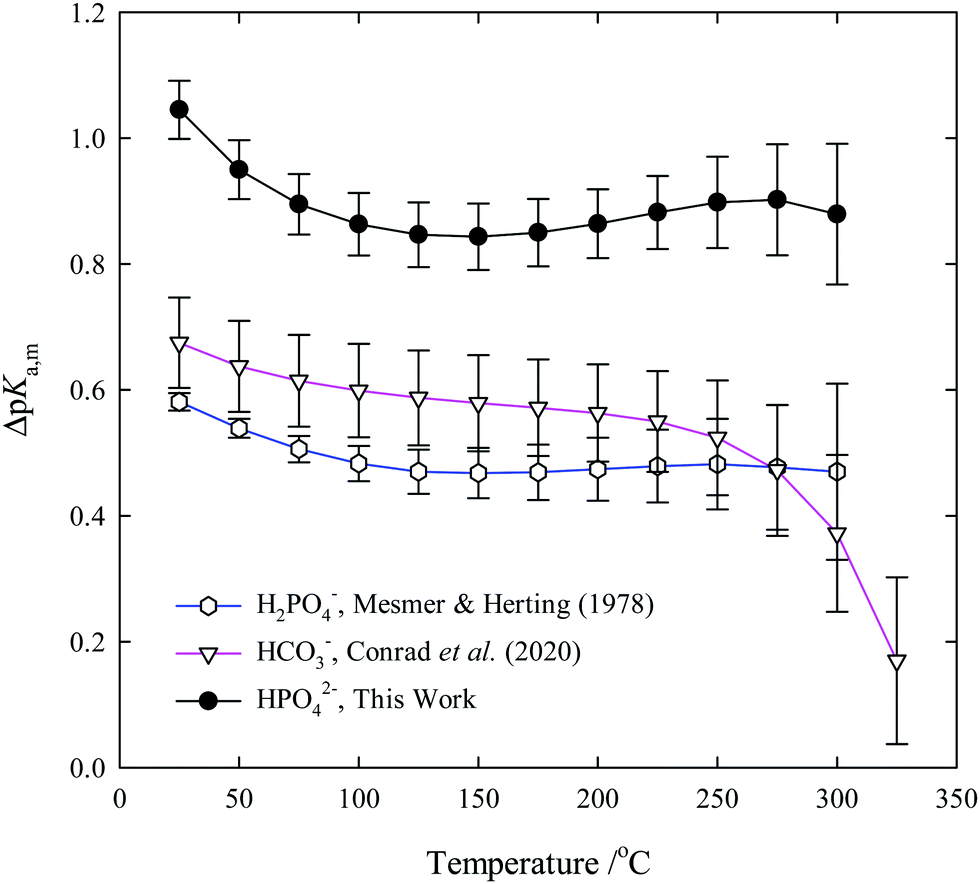
Third dissociation constant of phosphoric acid in H 2 O and D 2 O from 75 to 300 °C at p = 20.4 MPa using Raman spectroscopy and a titanium-sapphire f ... -
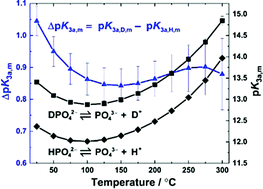
Third dissociation constant of phosphoric acid in H2O and D2O from 75 to 300 °C at p = 20.4 MPa using Raman spectroscopy and a titanium-sapphire flow cell - Physical Chemistry Chemical Physics (RSC Publishing)

Chemistry Laboratory: Neutralization of a polyprotic acid with a strong base (Key words: Phosphoric acid)