![The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ] The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]](https://haygot.s3.amazonaws.com/questions/1938773_1231298_ans_7f19b0e0d221405fbf38b0df680608aa.jpg)
The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]

Calculate the pH of 0.1 M acetic acid solution if its dissociation constant is 1.8 × 10^-5 . If 1 litre of this solution is mixed with 0.05 mole of HCl , what will be pH of the mixture?
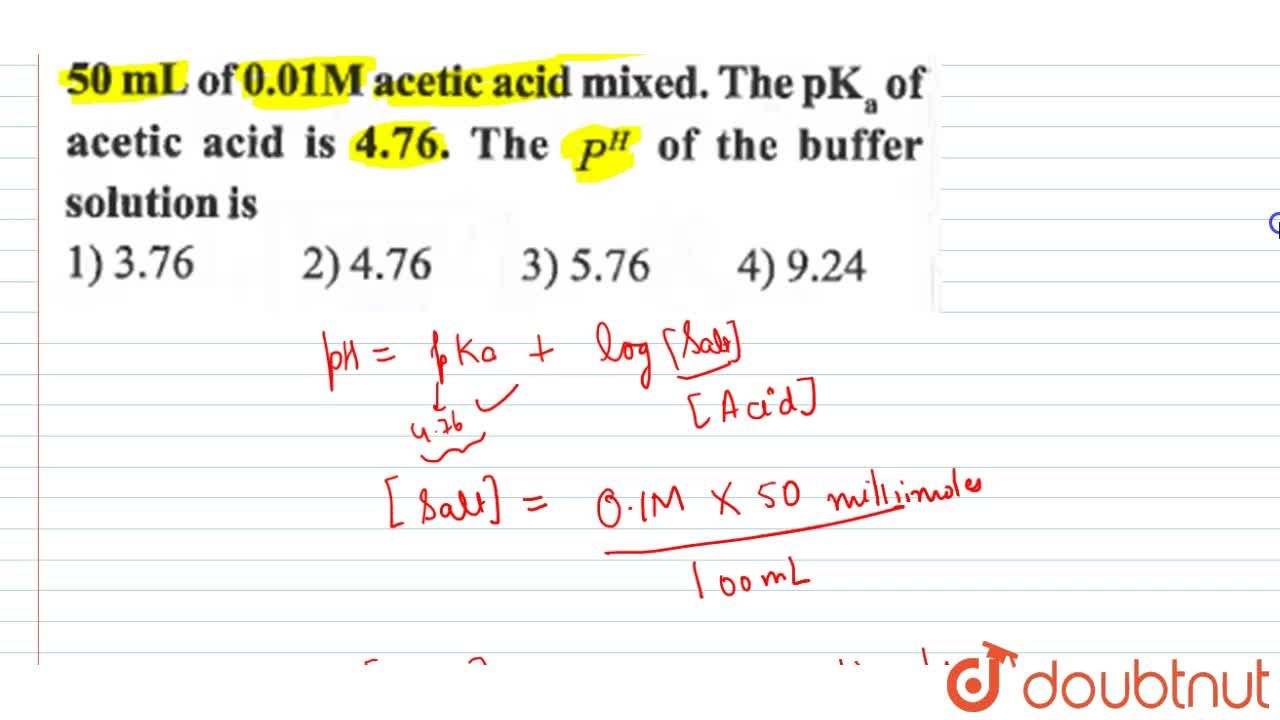
50 mL of 0.1 M solution of sodium acetate and 50 mL of 0.01 M acetic acid mixed. The pK(a) of acetic acid is 4.76. The P^(H) of the buffer solution is

To a solution of 20 mL of 0.1 M acetic acid, a solution of 0.1 M NaOH is added from burette. If 'r' - YouTube

Calculate ph of 0.1m solution of acetic acid if degree of dossocuation of the acid us 0.0132 - Brainly.in

Calcualte the pH at the equivalence point when a solution of 0.1M acetic is titrated with a solution of 0.1M NaOH.(K(a)for acid = 1.9xx10^(-5)).
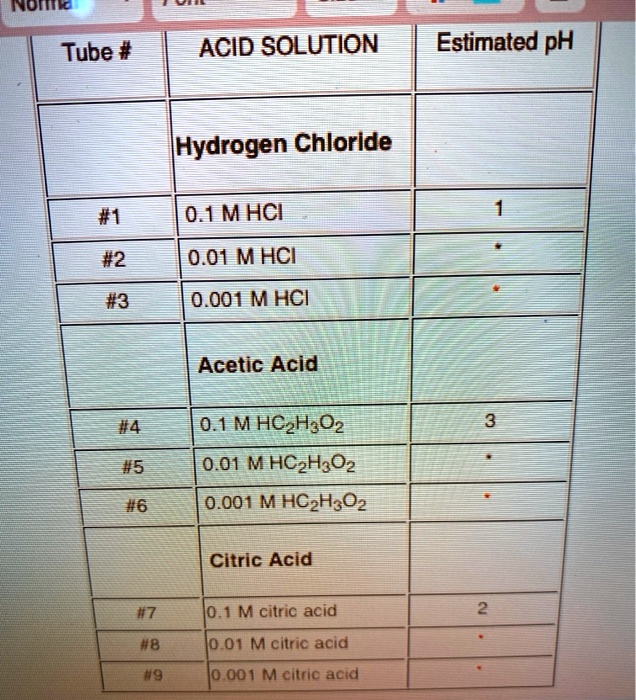
SOLVED: Inomo Tube # ACID SOLUTION Estimated pH [Hydrogen Chloride #1 0.1MHCI #2 #3 0.01 MHCI 0.001 MHCI Acetic Acid #4 0.1MHCzH;Oz 0.01 M HCzH3Oz 0.001 M HCzH3Oz #5 #6 Citric Acid
What is the pH of 0.1 m of acetic solution, if acetic acid is a weak acid with Ka2 1.86 × 10-5? - Quora

SOLVED: 4S - -(25 6: Find the pH values for the following solutions 0.1 M HCL b. 0. M NaOH 0.05 M HCL d. 0.05 M NaOH 8: What is the pH ofa 0.1 M solution of acetic acid (pKa= 4.75)?