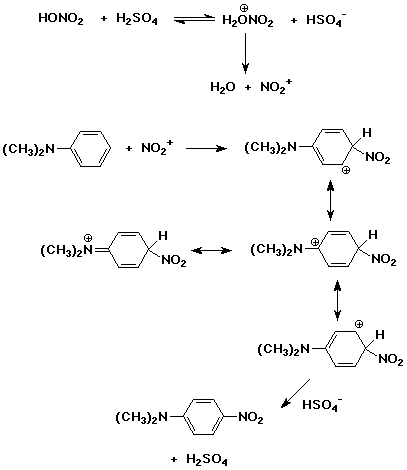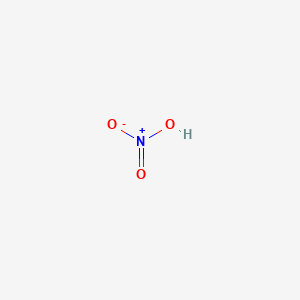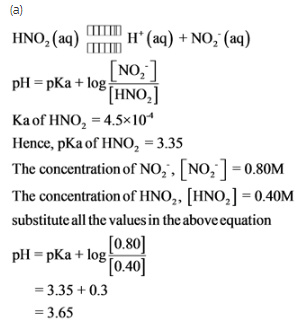
Nitric acid, HNO2 is a weak acid with Ka = 4.5 x 10-4. It dissociates according to: HNO2 (aq) - Home Work Help - Learn CBSE Forum

Topic 18- Acids and bases 18.1 Calculations involving acids and bases 18.2 Buffer solutions 18.3 Salt hydrolysis 18.4 Acid-base titrations 18.5 Indicators. - ppt video online download
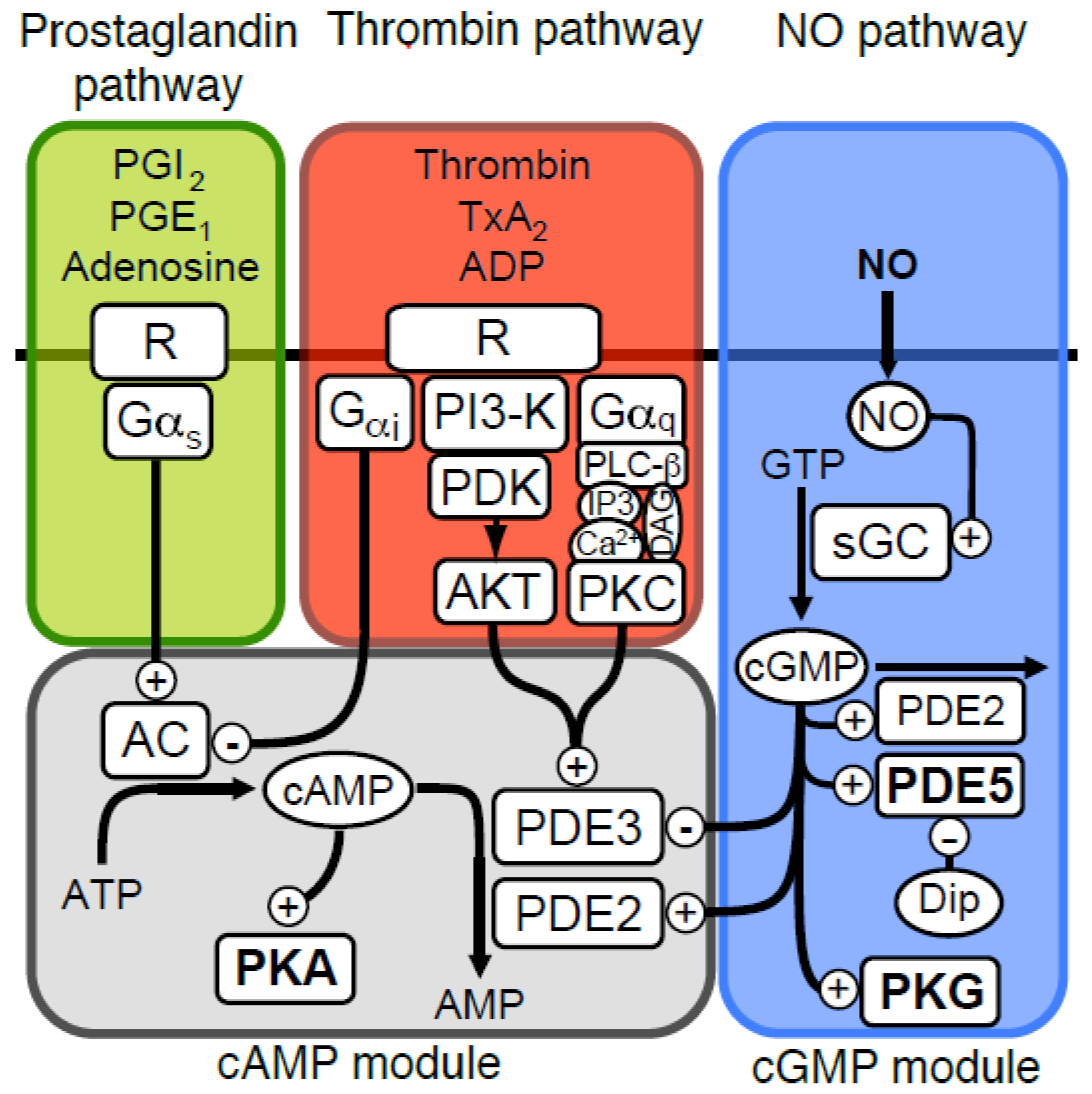
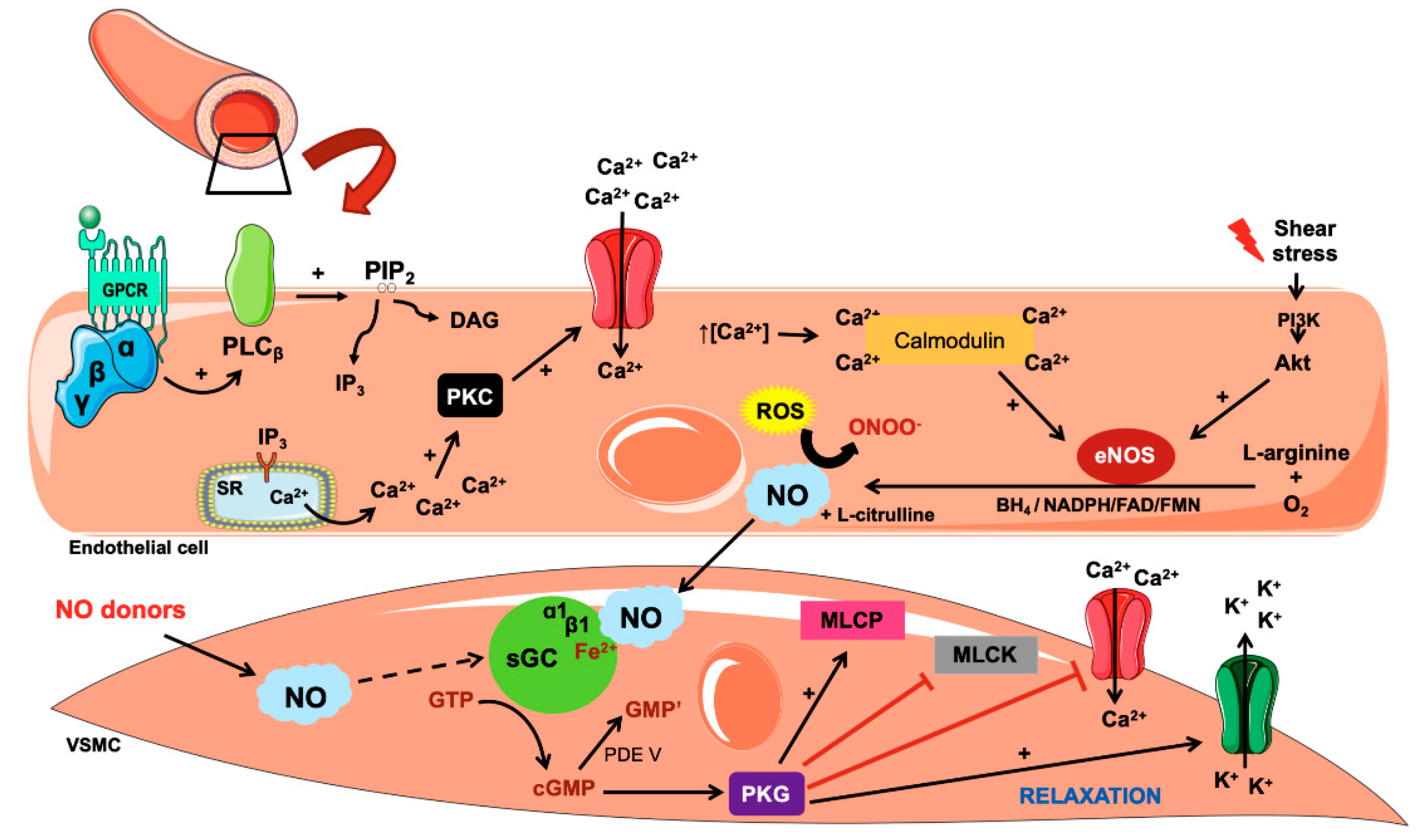
IJMS | Free Full-Text | Mathematical Modelling of Nitric Oxide/Cyclic GMP/Cyclic AMP Signalling in Platelets
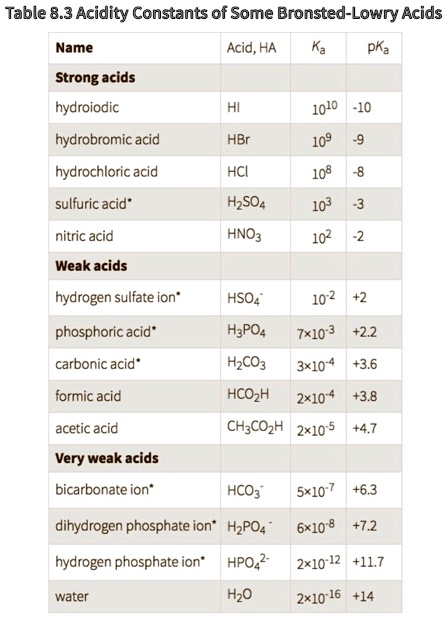
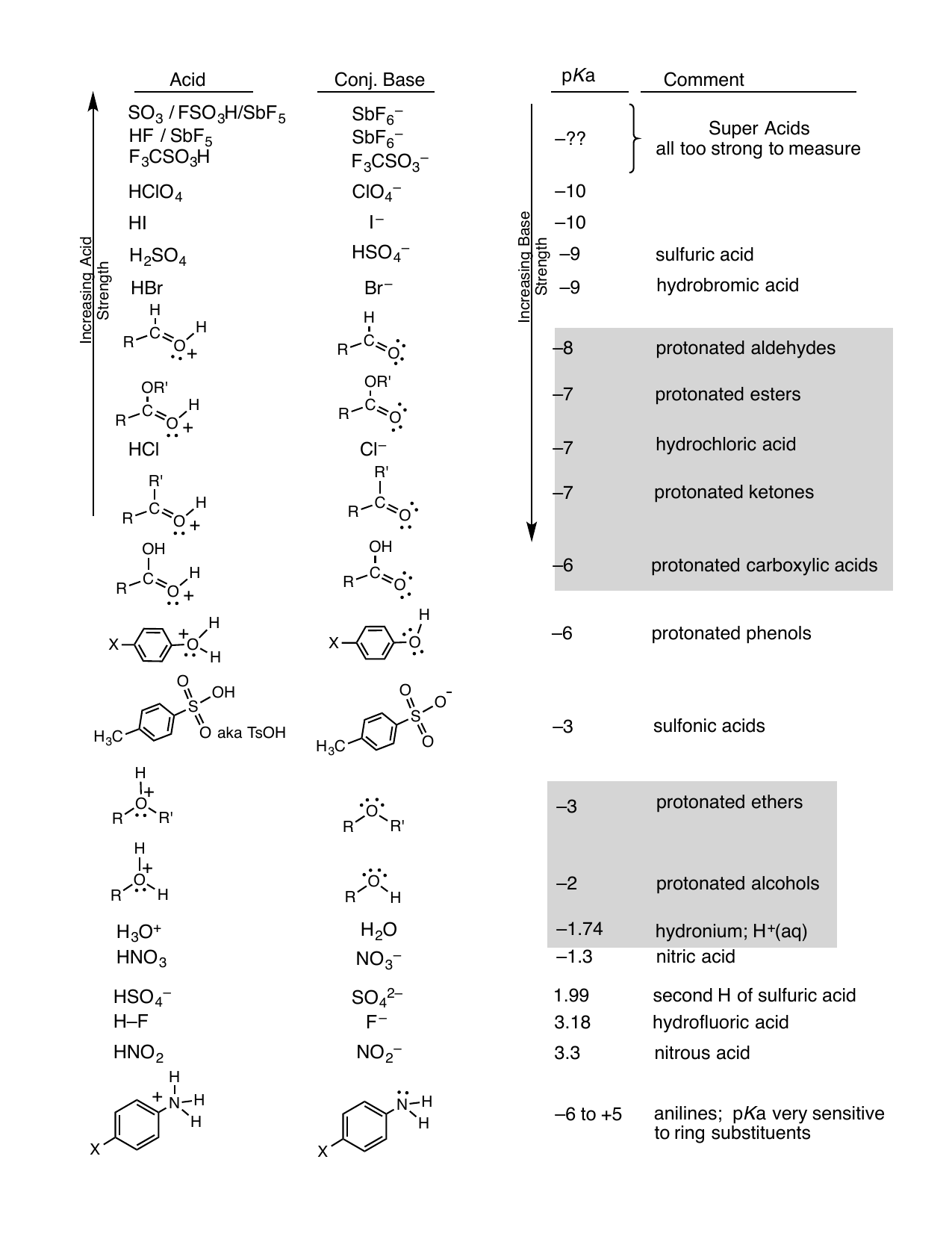
SOLVED: Table 8.3 Acidity Constants of Some Bronsted-Lowry Acids Name Acid PKa Strong acids hydroiodic 1010 -10 hydrobromic acid HBr 109 hydrochloric acid HCI 108 sulfuric acid* HzSO4 103 nitric acid HNO3

Expression of nitric oxide synthase (NOS) in Anabas testudineus ovary and participation of nitric oxide-cyclic GMP cascade in maintenance of meiotic arrest - ScienceDirect

Cyclic AMP-dependent phosphorylation of neuronal nitric oxide synthase mediates penile erection | PNAS

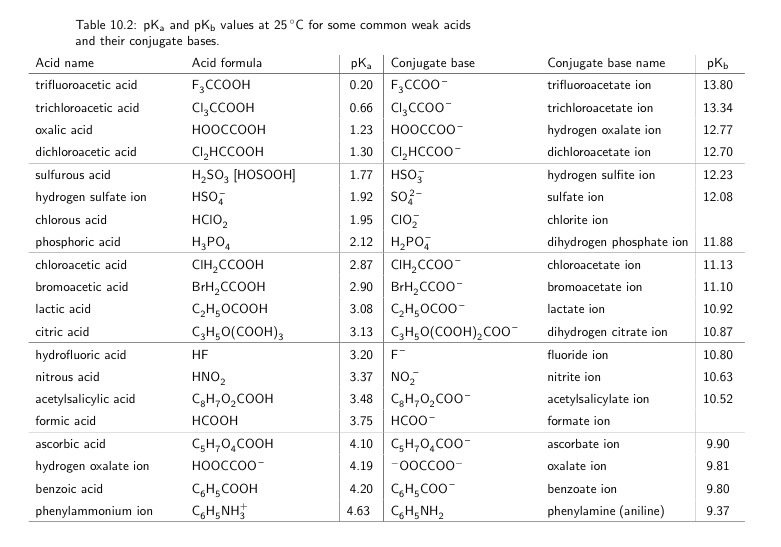
pKa Ka data factors affecting Acidic reactions of carboxylic acids with metals oxides hydroxides carbonates hydrogencarbonate test advanced A level organic chemistry revision notes doc brown

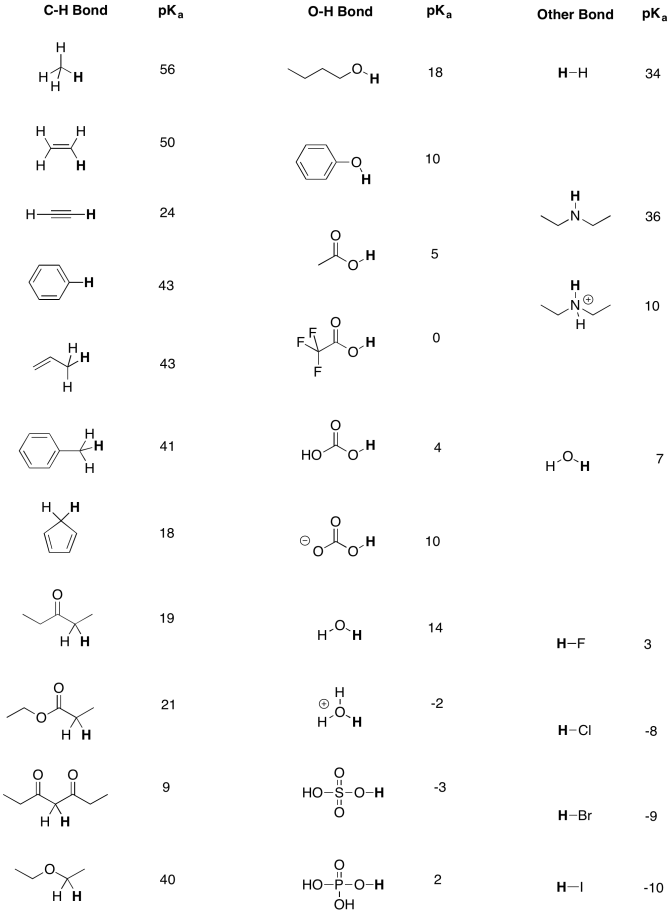
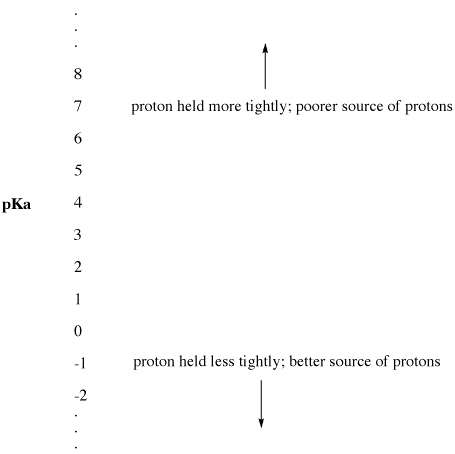
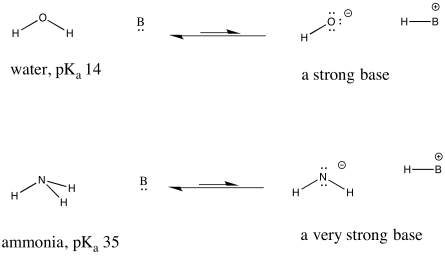
organic chemistry - Why Does A Brønsted–Lowry Acid Accept Proton from Stronger Acid? - Chemistry Stack Exchange