
H2CrO4 and Cr2O7 2-binding energy with 3d transition metals (Sc, Ti,... | Download Scientific Diagram

Consider a spontaneous electrochemical cell between Cu and Al . Predict what would happen if excess concentrated NaOH were added to the cell with copper ions and a precipitate forms.Standard Potential (V)Reduction
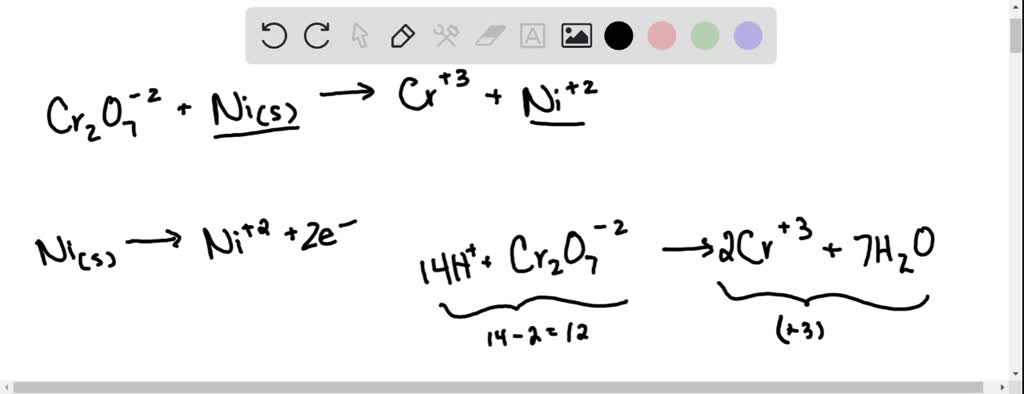
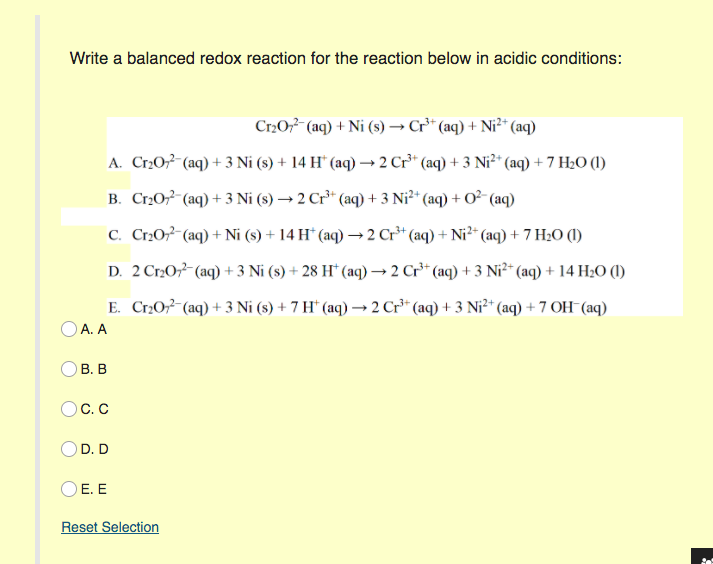
SOLVED: Balance the following redox equation in an acidic solution : Cr2O7 -2 (aq) + Ni (s) —– > Ni2+ (aq) + Cr3+ (aq) (acidic solution)

H2CrO4 and Cr2O7 2-binding energy with 3d transition metals (Sc, Ti,... | Download Scientific Diagram

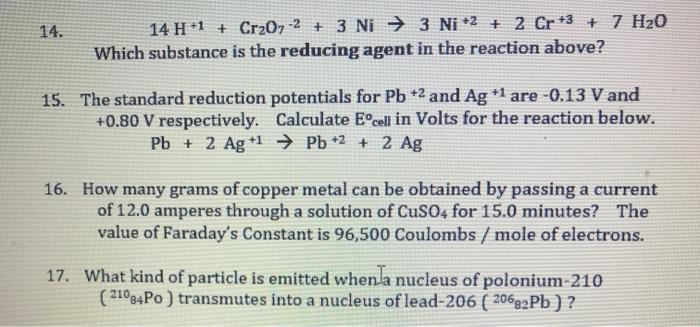
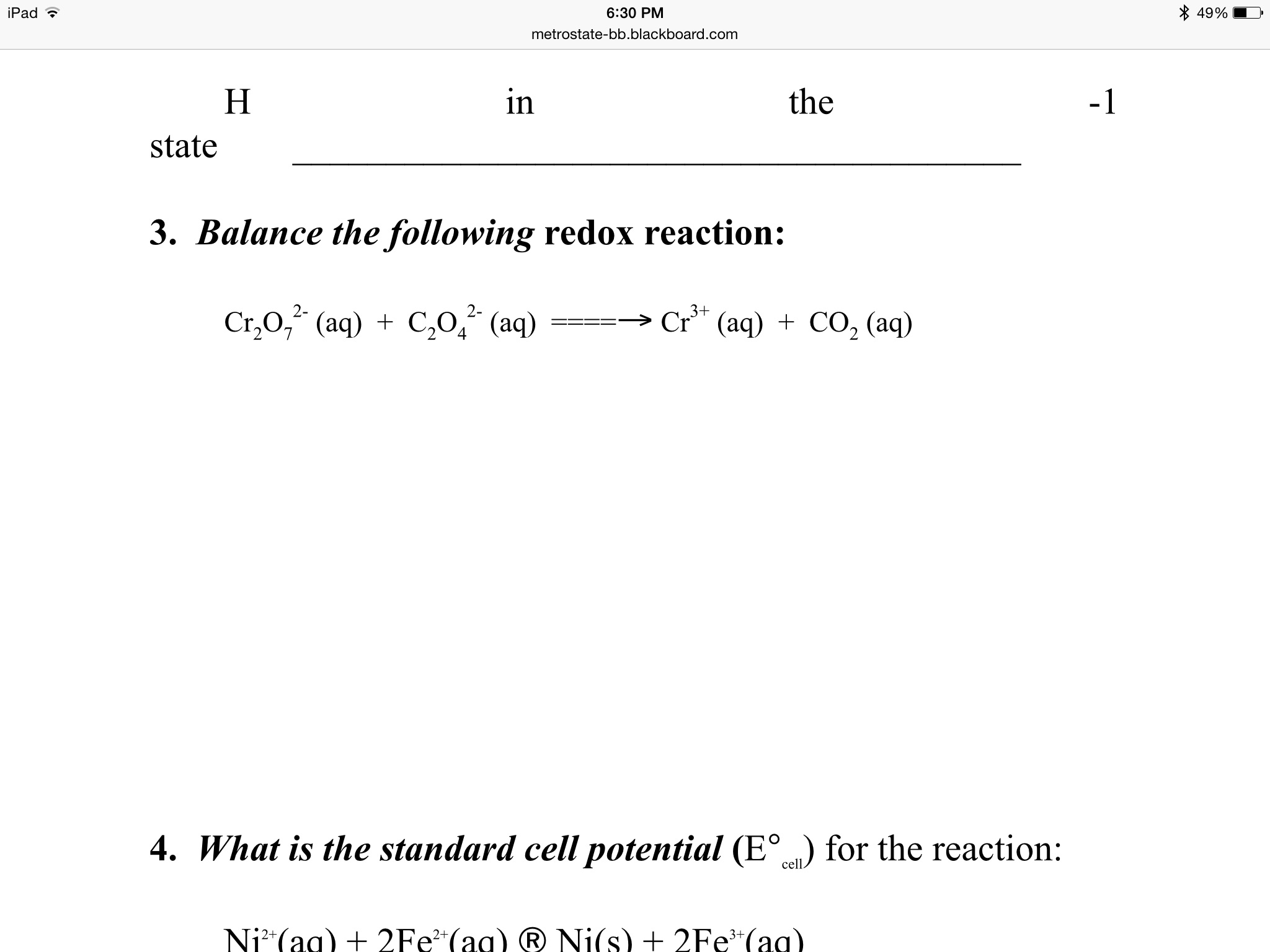
For the redox reaction Cr(2)O(7)^(-2)+H^(+)+Ni rarr Cr^(3)+Ni^(2+)+H(2)O The correct coefficients of the reactions for the balanced reaction are
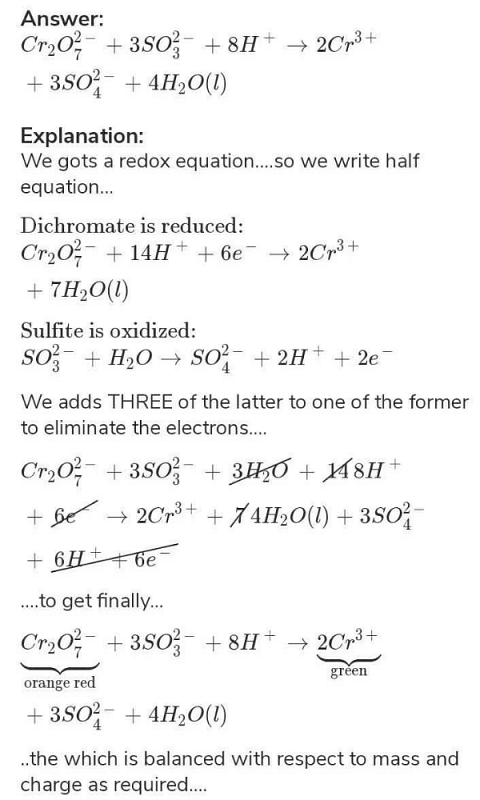
Balance the following ionic equations (i) Cr2O7^2-+H^++I^- → Cr^3+ +I2+H2O - Sarthaks eConnect | Largest Online Education Community
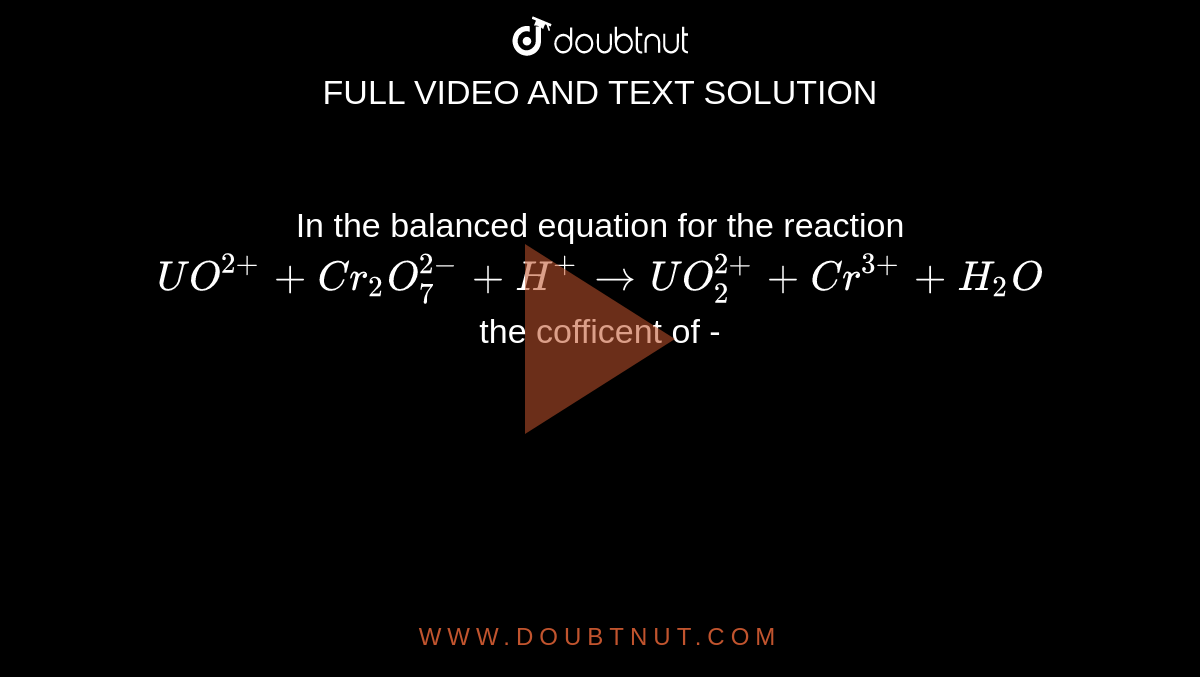
For the redox reaction Cr(2)O(7)^(-2)+H^(+)+Ni rarr Cr^(3)+Ni^(2+)+H(2)O The correct coefficients of the reactions for the balanced reaction are

Chromium transition metal Chemistry chromium(III) Cr3+ complex ions chromate(VI) CrO42- dichromate(VI)Cr2O72- redox chemical reactions principal +3 +6 oxidation states ligand substitution GCE AS A2 IB A level inorganic chemistry revision notes
![Novel mode of 2-fold interpenetration observed in a primitive cubic network of formula [Ni(1,2-bis(4-pyridyl)acetylene)2(Cr2O7)]n - Chemical Communications (RSC Publishing) Novel mode of 2-fold interpenetration observed in a primitive cubic network of formula [Ni(1,2-bis(4-pyridyl)acetylene)2(Cr2O7)]n - Chemical Communications (RSC Publishing)](https://pubs.rsc.org/en/Content/Image/GA/C5CC05866J)
Novel mode of 2-fold interpenetration observed in a primitive cubic network of formula [Ni(1,2-bis(4-pyridyl)acetylene)2(Cr2O7)]n - Chemical Communications (RSC Publishing)




![Polar Compounds Constructed with the [Cr2O7]2- Anion | Inorganic Chemistry Polar Compounds Constructed with the [Cr2O7]2- Anion | Inorganic Chemistry](https://pubs.acs.org/cms/10.1021/ic000953y/asset/images/medium/ic000953yn00001.gif)
![Ni(H2O)6]2(Cr2O7)2(hmta)4•2H2О (HMTA - hexamethylenetetramine) : r/chemistry Ni(H2O)6]2(Cr2O7)2(hmta)4•2H2О (HMTA - hexamethylenetetramine) : r/chemistry](https://i.redd.it/c6l3dusld2471.jpg)
![Polar Compounds Constructed with the [Cr2O7]2- Anion | Inorganic Chemistry Polar Compounds Constructed with the [Cr2O7]2- Anion | Inorganic Chemistry](https://pubs.acs.org/cms/10.1021/ic000953y/asset/images/large/ic000953yn00001.jpeg)
![Cr2O7]2- - Dichromate Cr2O7]2- - Dichromate](http://www.chemtube3d.com/images/gallery/PNGfiles%20structures/I619STB1.png)
