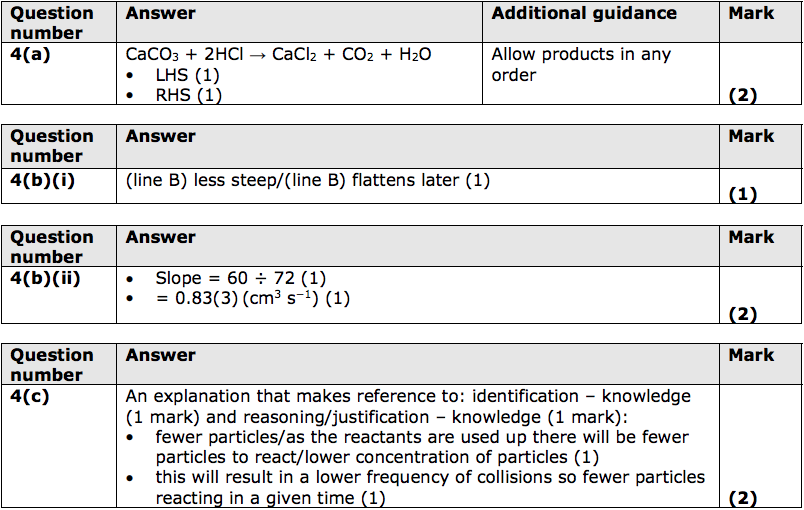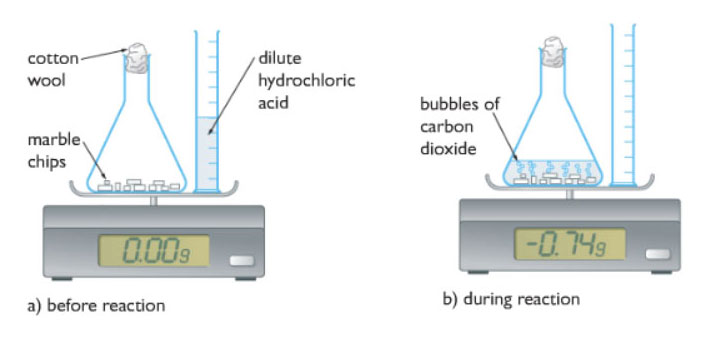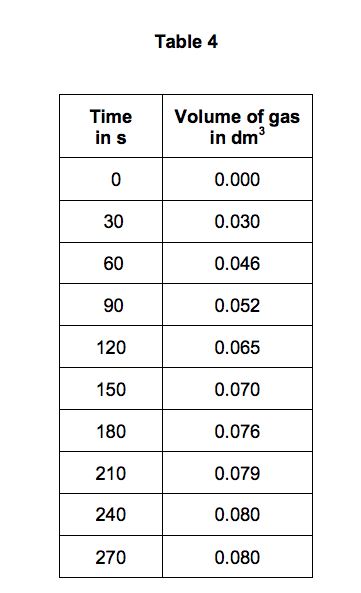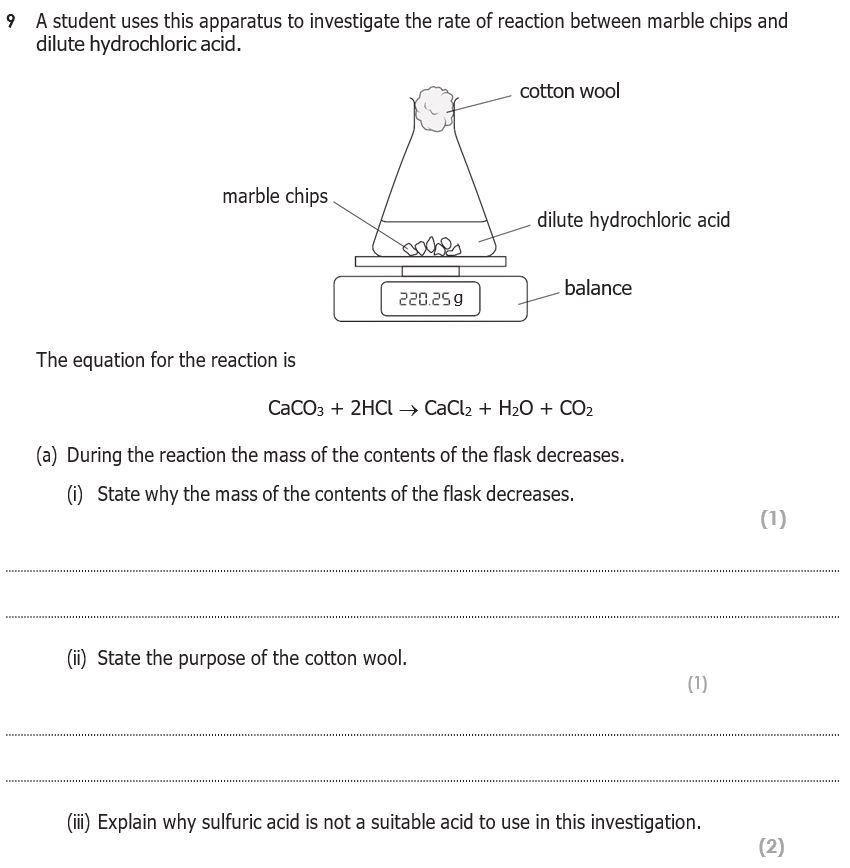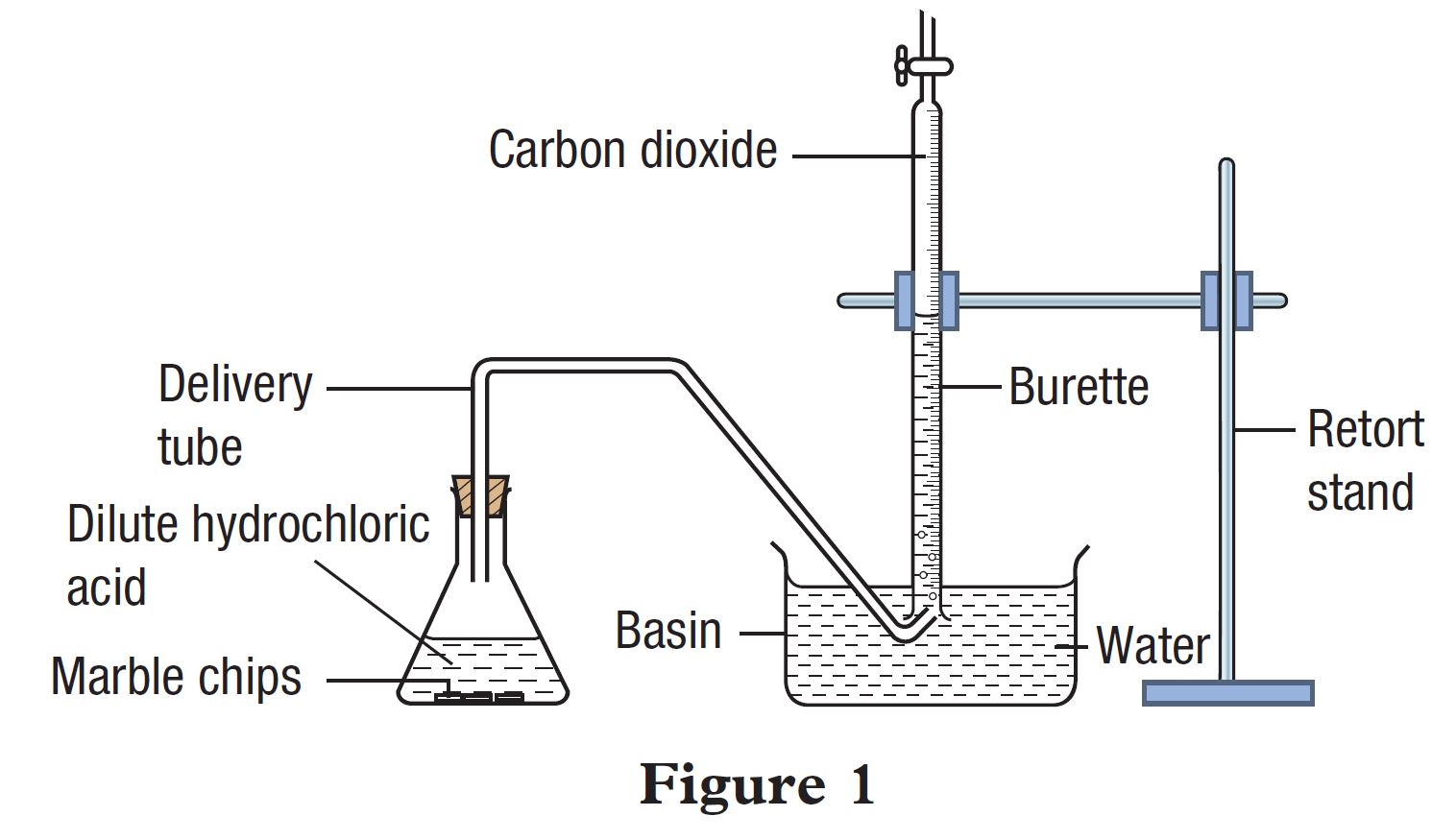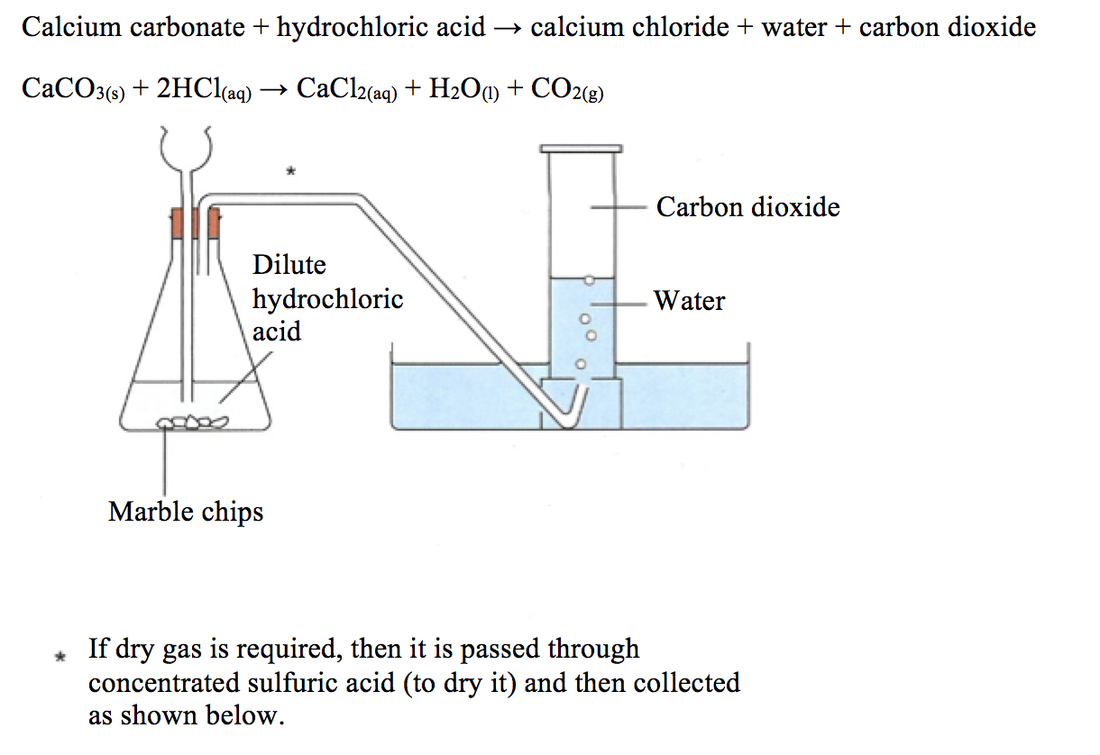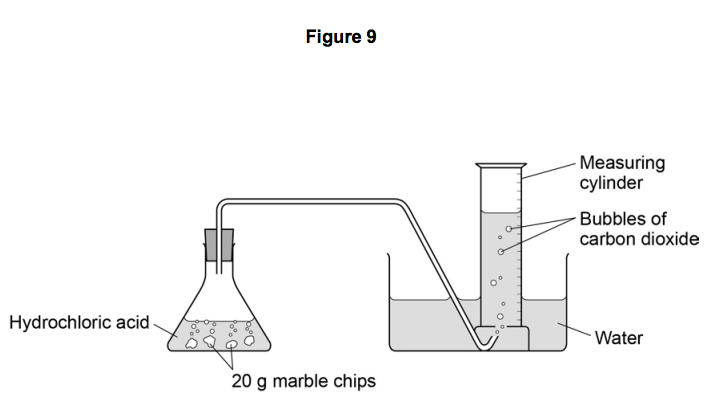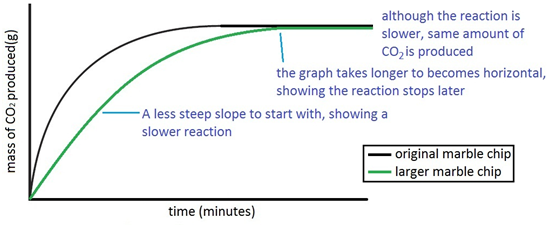
3:15 practical: investigate the effect of changing the surface area of marble chips and of changing the concentration of hydrochloric acid on the rate of reaction between marble chips and dilute hydrochloric
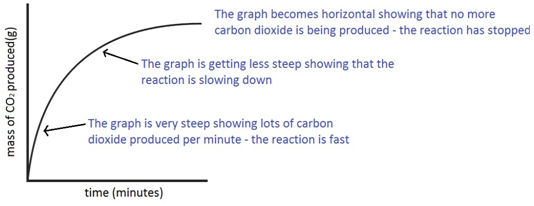
3:15 practical: investigate the effect of changing the surface area of marble chips and of changing the concentration of hydrochloric acid on the rate of reaction between marble chips and dilute hydrochloric

a student was investigating the reaction between marble chips and dilute hydrochloric acid - Brainly.com

Powder marble reacts with hydrochloric acid using the apparatus shown.The gas syringe fills in 36 seconds.The experiment is repeated using marble chips in place of powdered marble.How long it take to fill
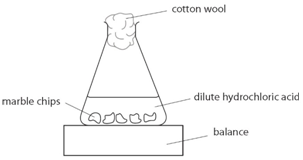
3:15 practical: investigate the effect of changing the surface area of marble chips and of changing the concentration of hydrochloric acid on the rate of reaction between marble chips and dilute hydrochloric
A solution of Y was slowly added to a solution of X. The graph shows how the pH of the resulting solution changed. (a) (i)

Practical: Effect of Surface Area on Rate of Reaction (3.2.5) | Edexcel IGCSE Chemistry Revision Notes 2019 | Save My Exams