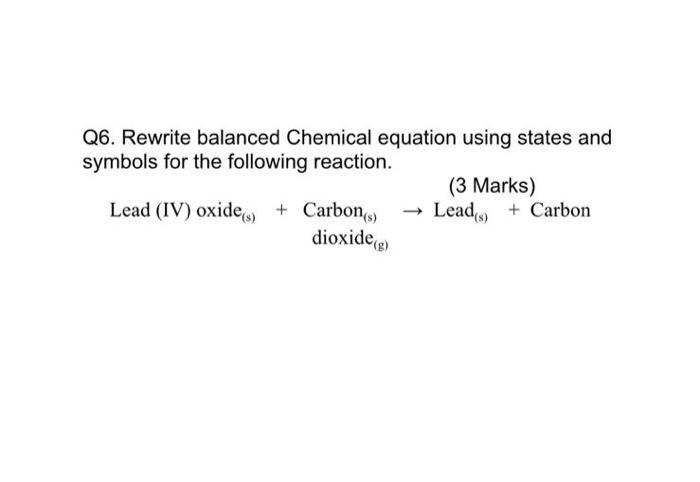
Quiz (Extraction of Metals) 1. Metals are usually extracted from their ores before use. State the method of extraction for the f

Marie mixed 5g of carbon with 5g of lead oxide. she heated the mixture strongly for 15 minutes in a - Brainly.com

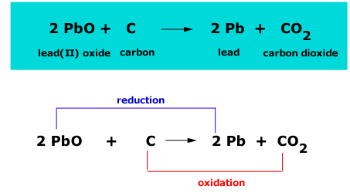
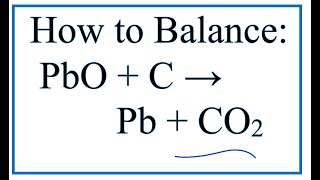
Which of the statements about the reaction below are incorrect ? 2PbO(s) + C(s) → 2Pb(s) + CO2(g) (a) Lead is getting reduced.(b) Carbon dioxide is getting oxidised.(c) Carbon is getting oxidised.(d)

Which of the statements about the reaction below are incorrect ? 2PbO(s) + C(s) → 2Pb(s) + CO2(g) (a) Lead is getting reduced.(b) Carbon dioxide is getting oxidised.(c) Carbon is getting oxidised.(d)
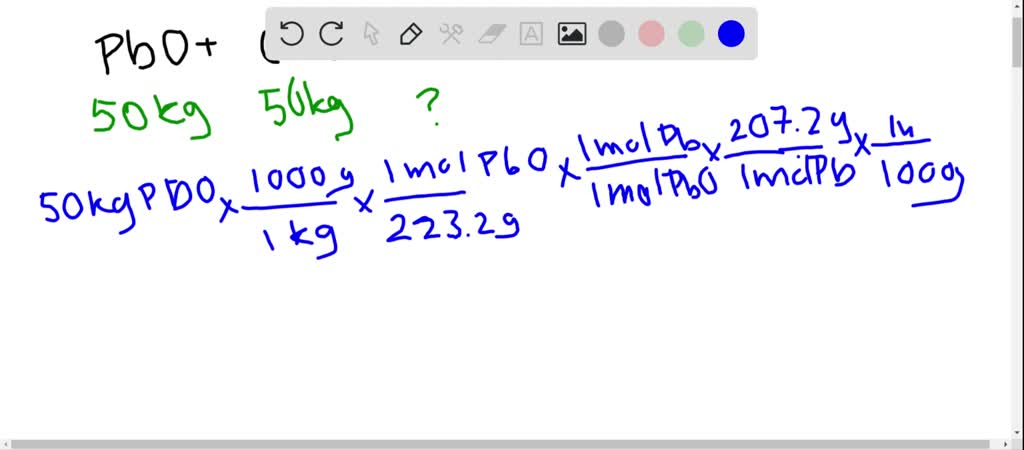
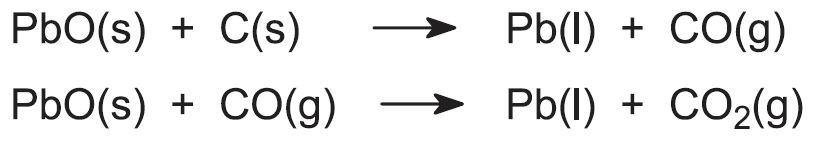
SOLVED:Lead(II) oxide from an ore can be reduced to elemental lead by heating in a furnace with carbon. PbO(s)+C(s) →Pb(l)+CO(g) Calculate the expected yield of lead if 50.0 kg of lead oxide

SOLVED: Lead (II) carbonale decomposes t0 give lead (IIl) oxide and carbon dioxide: PbCO3 (s) + PbO (s) COz (g) grams of lead (II) oxide will be produced by the decomposition of