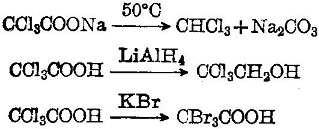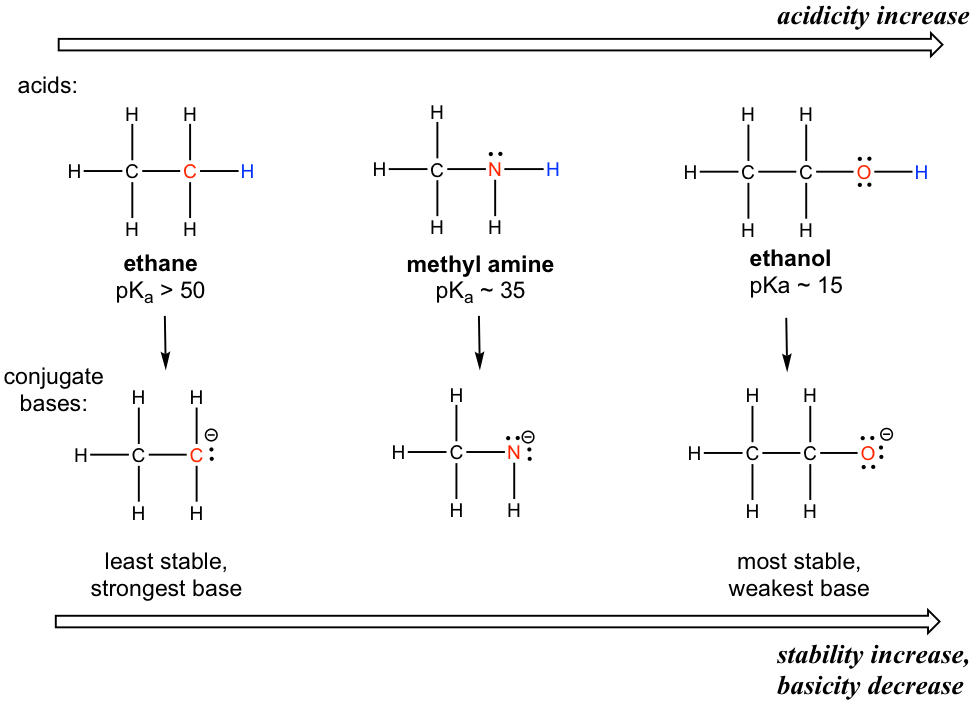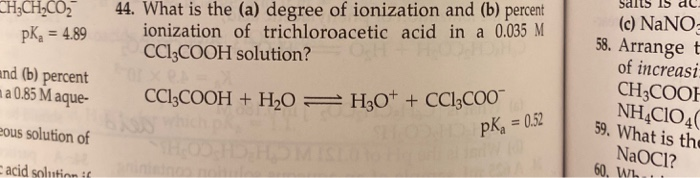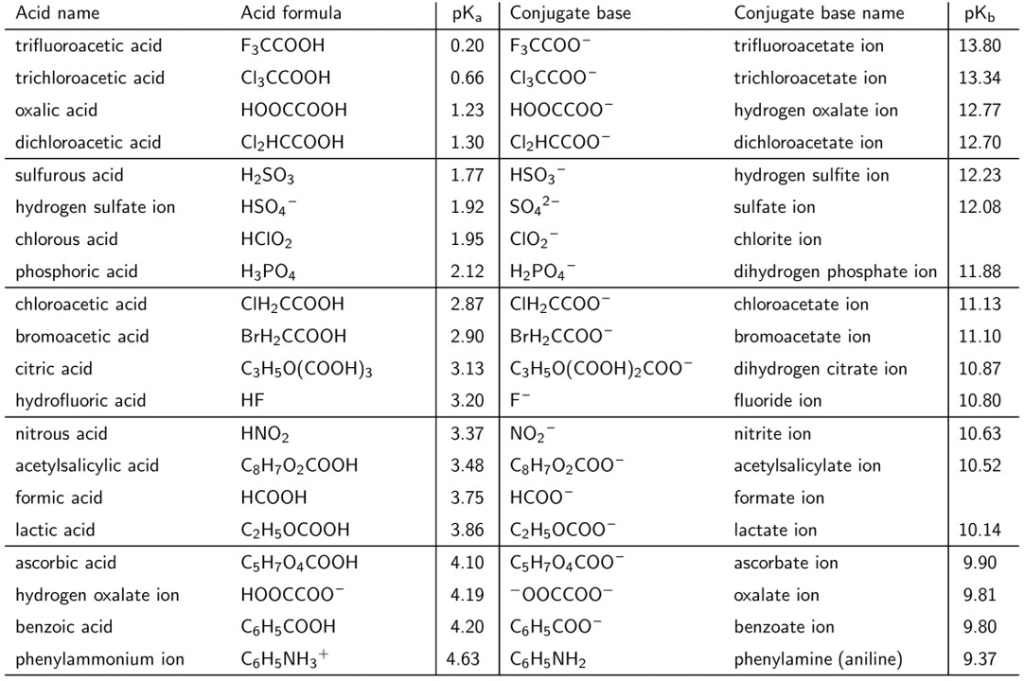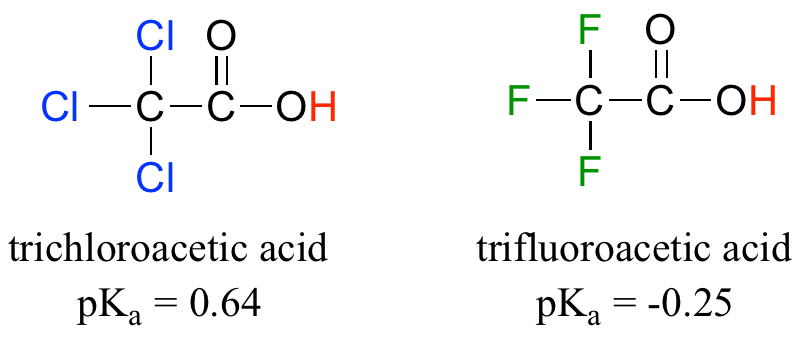
Fluorescence enhancement of quinolines by protonation - RSC Advances (RSC Publishing) DOI:10.1039/D0RA04691D

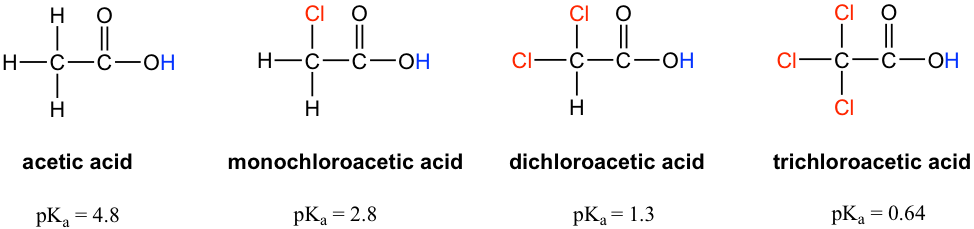
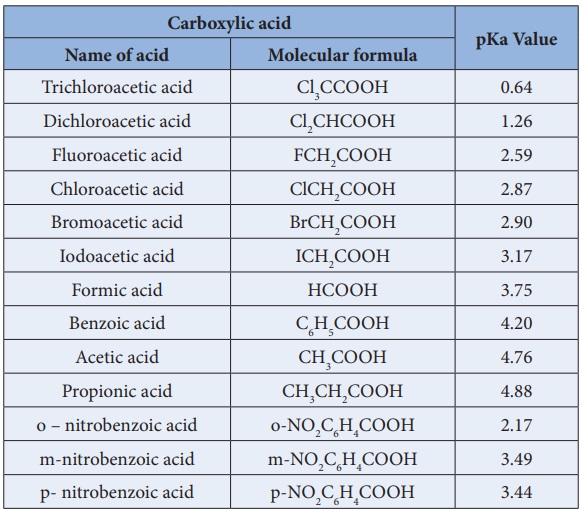
Chloroacetic acid is a stronger acid than acetic acid. Give Reason. | CurlyArrows Chemistry Tutorials

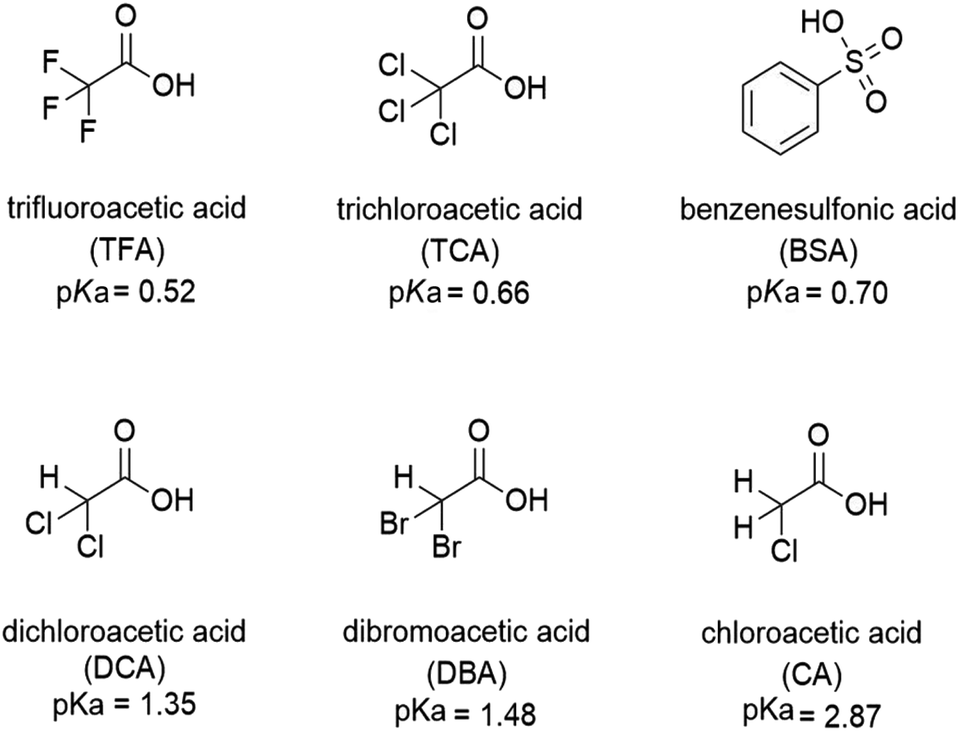
How to determine the strength of facial peels: debunking the myths and to determine how strong different peelings really are - nunii

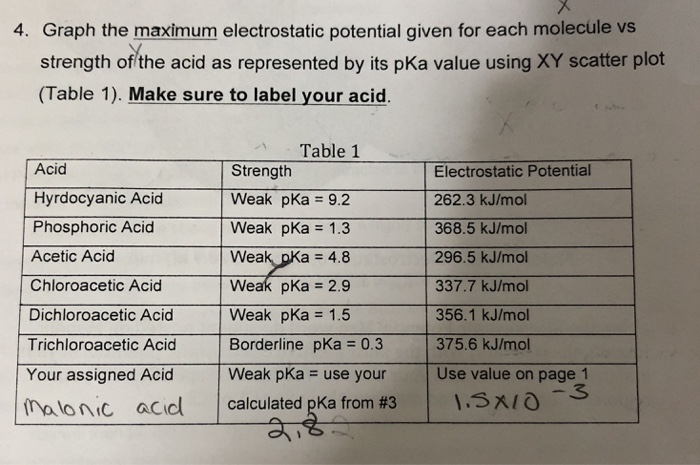
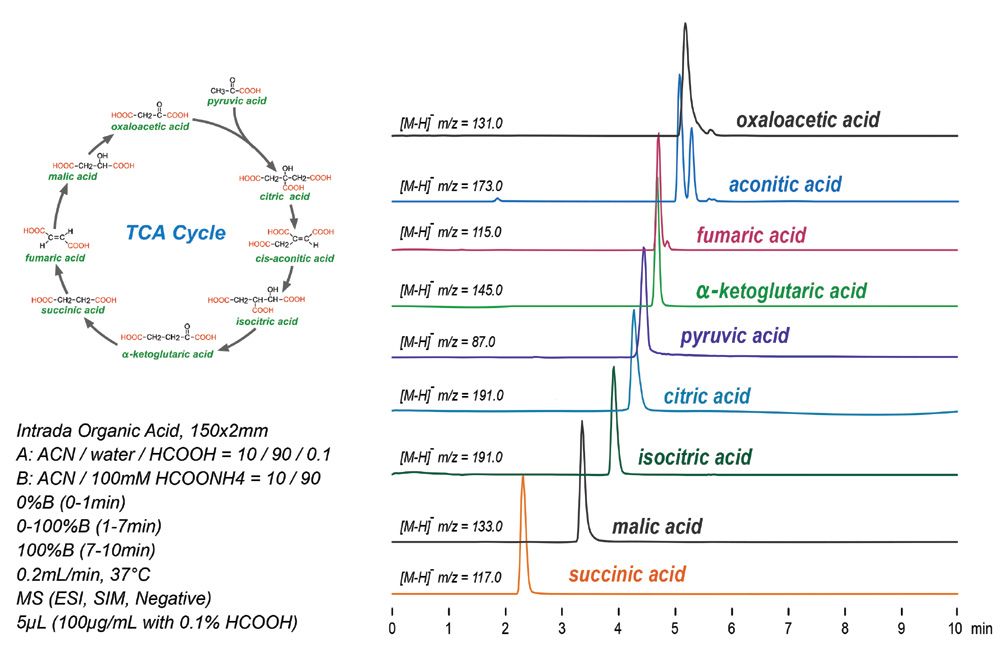
A Reliable and Efficient First Principles-Based Method for Predicting pKa Values. 2. Organic Acids | The Journal of Physical Chemistry A