IVD Directive to IVD Regulation (EU 2017/746) Transition – 8 Months Remaining - Voisin Consulting Life Sciences
CD10 antibodies | CE-IVD reagents | Clinical flow cytometry | Cell manufacturing platform | Products | Miltenyi Biotec | Great Britain

Top 10 Questions regarding the UK Responsible Person and medical device/IVD registration with the MHRA

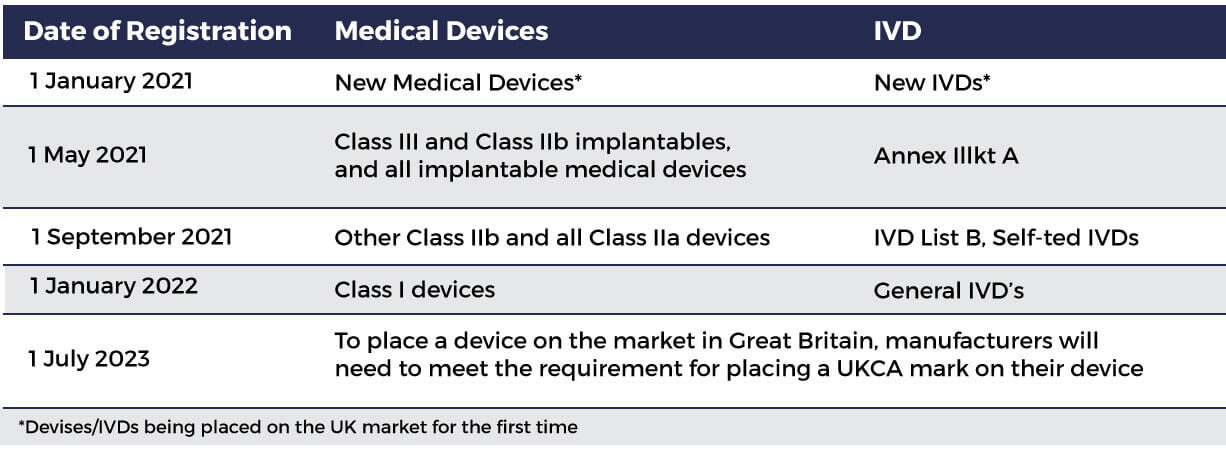
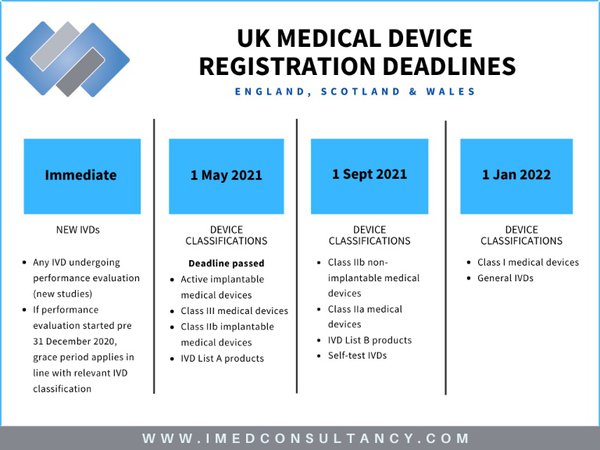
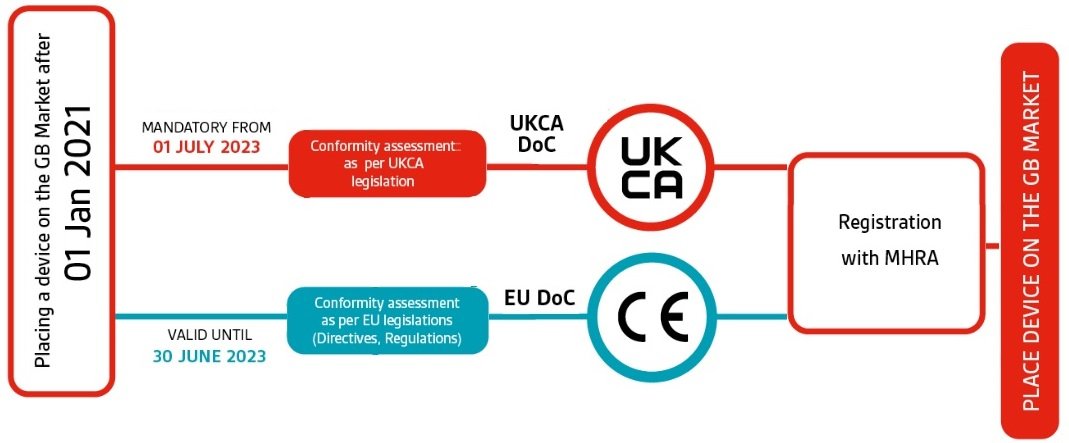
MHRA guidance on registration and deadlines for medical devices and IVDs in Great Britain and Northern Ireland

New UK medical device regulation spells potential trial concerns for some IVD players - Medical Device Network
Top 10 Questions regarding the UK Responsible Person and medical device/IVD registration with the MHRA