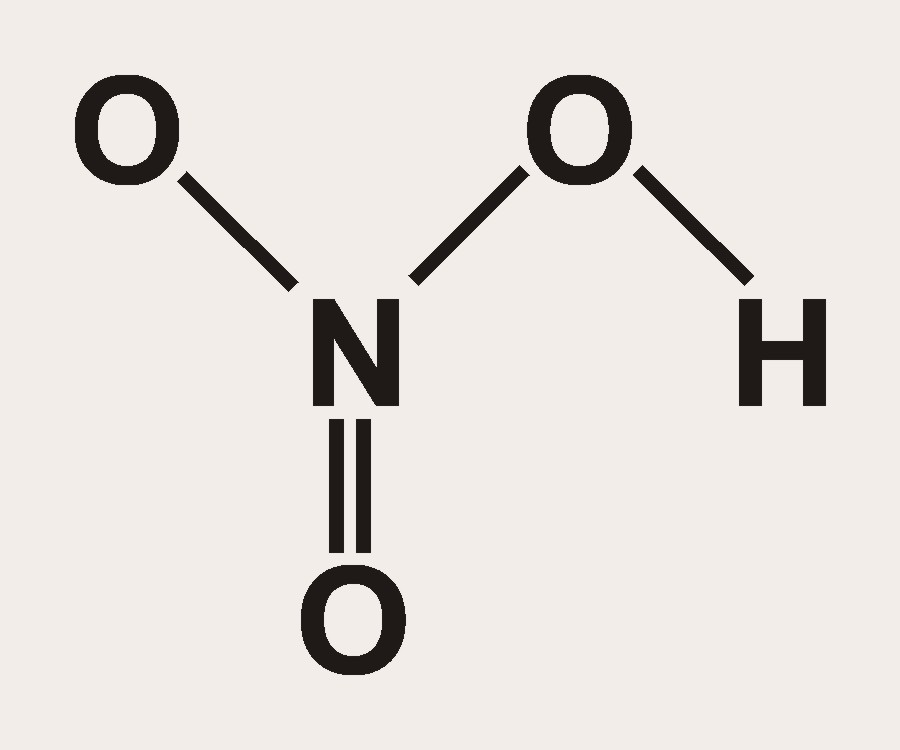
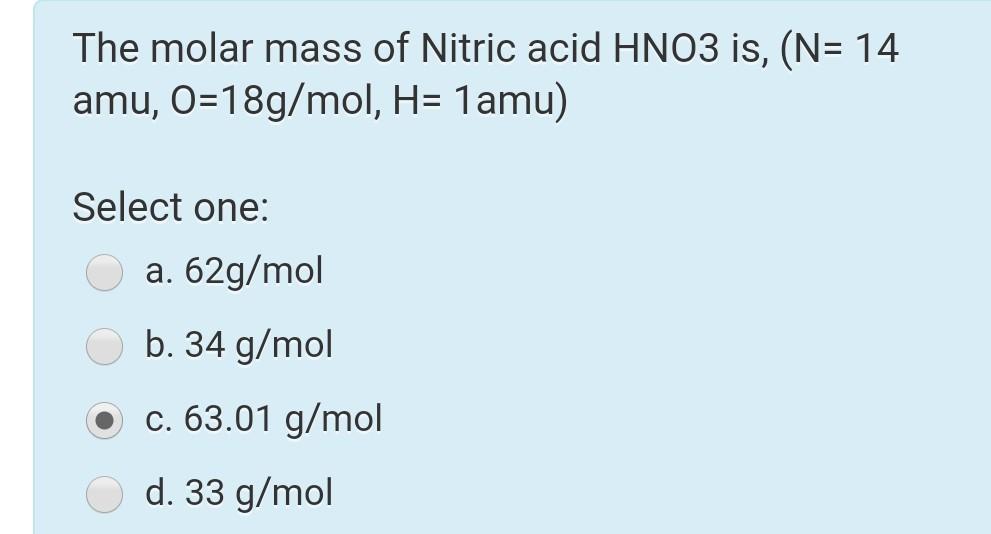
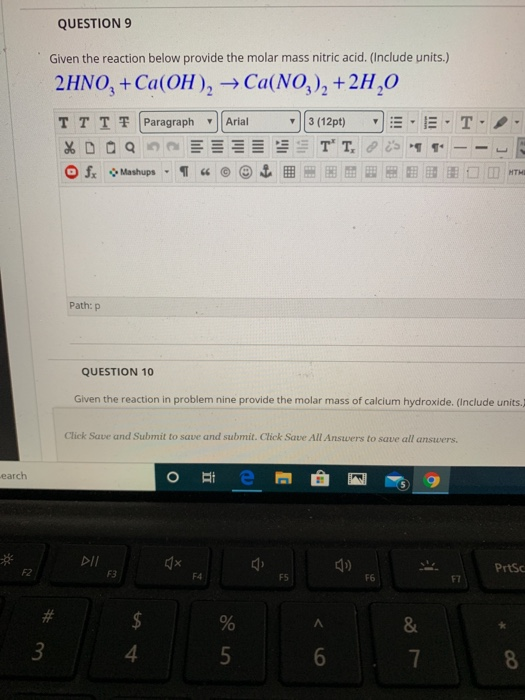
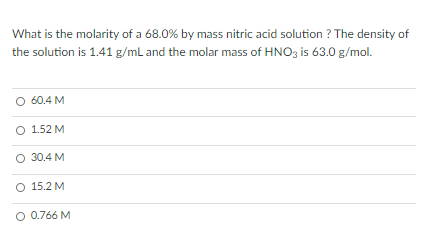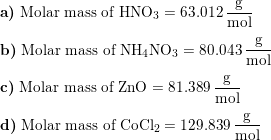How many grams of concentrated nitric acid solution should be used to prepare 250 mL of 2.0 M HNO3 ? The concentrated acid is 70

calculate the molar mass of the following Chemicals nitric acid (HNO3) explain with steps - Brainly.in

24. Calculate the molar mass of (a) water (H20) (b) nitric acid (HNO3).with full steps. - Brainly.in

Calculate the concentration of nitric acid in moles per litre in a sample which has density 1.41g/mL - YouTube
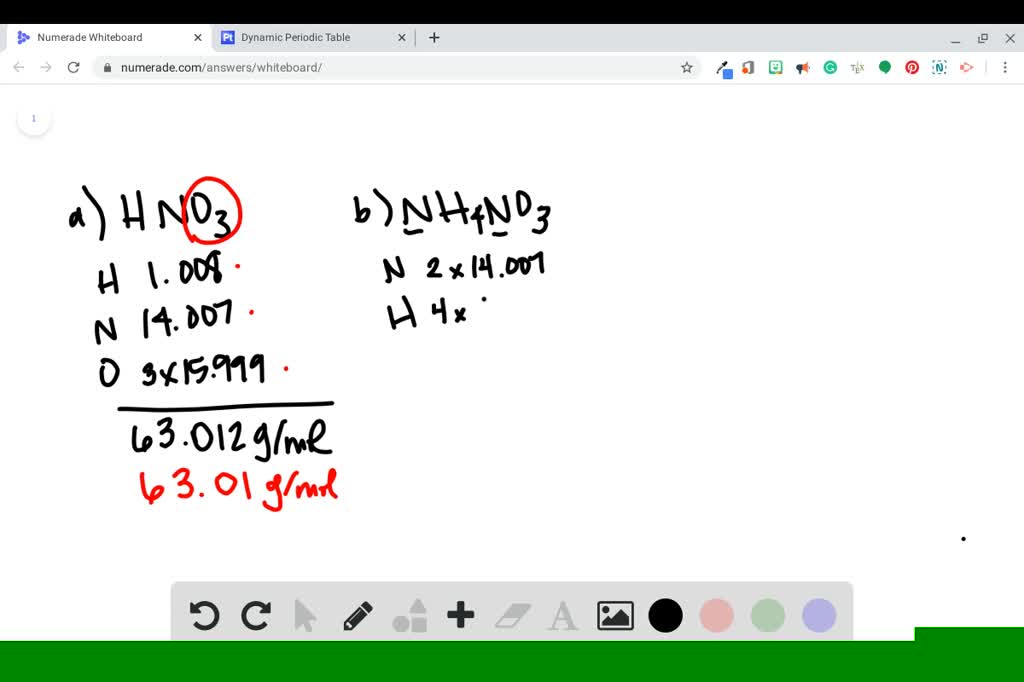
SOLVED:Determine the molar mass of each compound. a. nitric acid (HNO3) b. ammonium nitrate (NH4 NO3) c. zinc oxide (ZnO) d. cobalt chloride (CoCl2)

Calculate the concentration of nitric acid in moles per litre in a sample which has a density 1.41 g mL^-1 and the mass per cent of nitric acid in it being 69% .

Calculate the molecular mass of nitric acid, `HNO_(3)`. (Atomic masses :` H = 1 u, N = 14 u , - YouTube

100cm³ of 2M nitric acid reacted with 12.5g of a carbonate of metal M. (MCO<sub>3</sub>) according to the following equation (video) | Mole Concept | Kenyaplex - 172
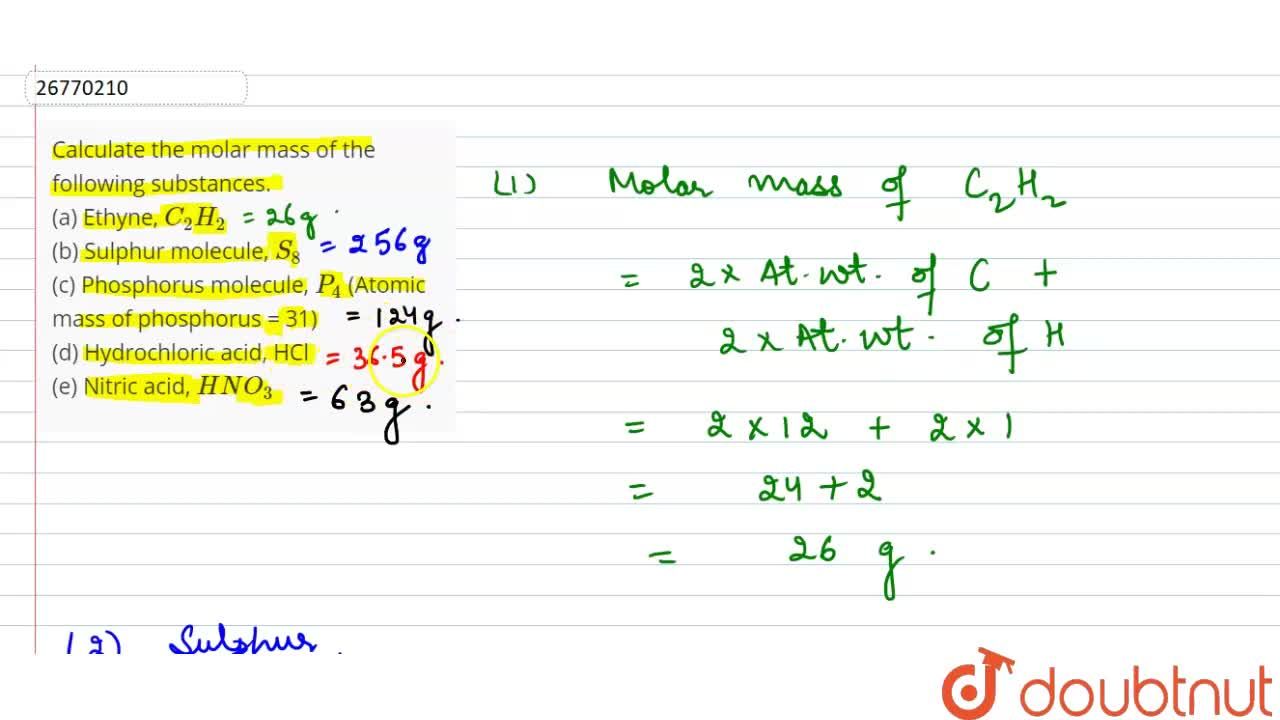
Calculate the molar mass of the following substances. (a) Ethyne, C(2)H(2) (b) Sulphur molecule, S(8) (c) Phosphorus molecule, P(4) (Atomic mass of phosphorus = 31) (d) Hydrochloric acid, HCl (e) Nitric acid,