Write the balanced equation for the reaction between a mixture of ammonium chloride and slaked lime.
![Why calcium ions do not form precipitate with aqueous ammonia [online video] – O Level Secondary Chemistry Tuition Why calcium ions do not form precipitate with aqueous ammonia [online video] – O Level Secondary Chemistry Tuition](https://icandochemistry942105908.files.wordpress.com/2021/10/qa-aq-ammonia.jpg?w=986)
Why calcium ions do not form precipitate with aqueous ammonia [online video] – O Level Secondary Chemistry Tuition
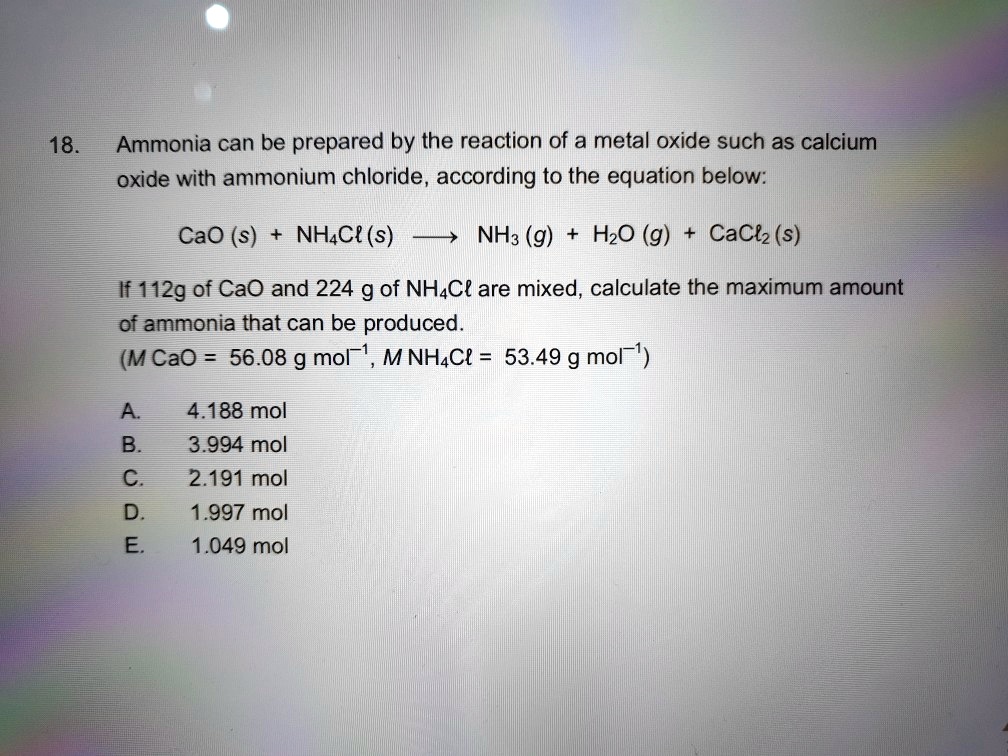
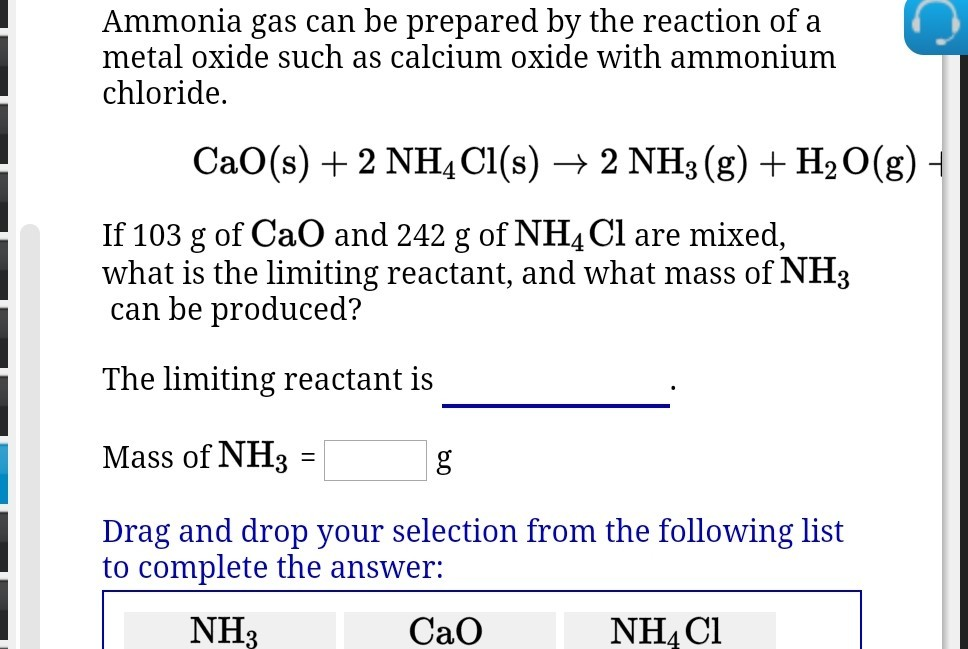
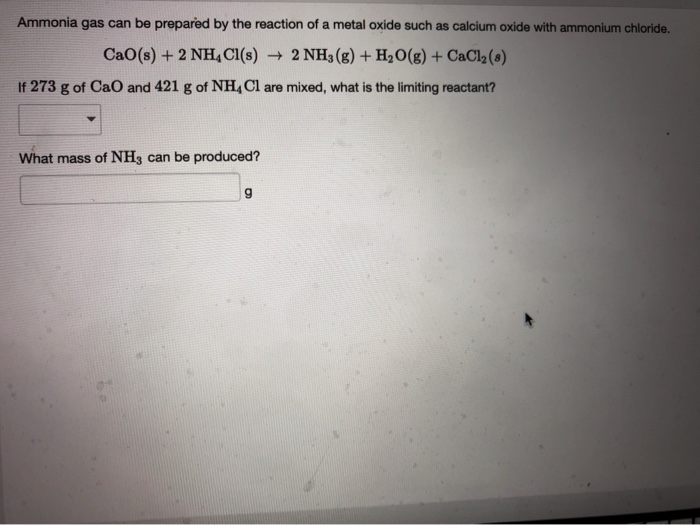
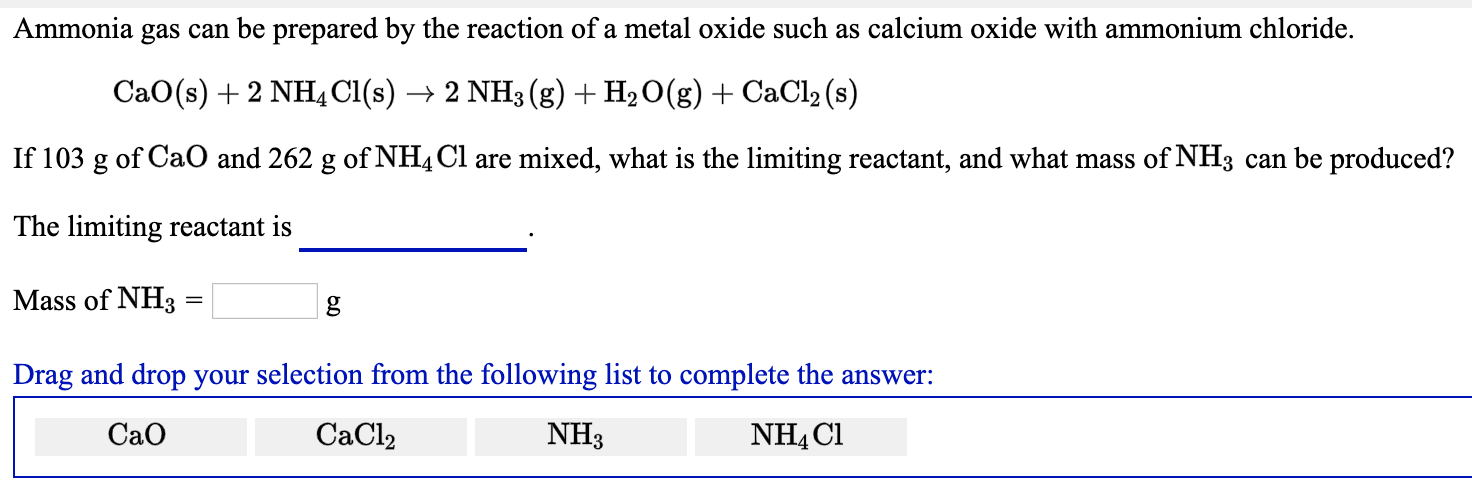
SOLVED: 18 Ammonia can be prepared by the reaction of a metal oxide such as calcium oxide with ammonium chloride , according to the equation below: CaO (s) NHAC? (s) NH: (g)

Preparation of Ammonia Gas in Laboratory with the Help of Ammonium Chloride and Calcium Oxide Stock Vector - Illustration of white, formula: 220304379
![Why calcium ions do not form precipitate with aqueous ammonia [online video] – O Level Secondary Chemistry Tuition Why calcium ions do not form precipitate with aqueous ammonia [online video] – O Level Secondary Chemistry Tuition](https://i.ytimg.com/vi/rXYJhFZTlTo/maxresdefault.jpg)
Why calcium ions do not form precipitate with aqueous ammonia [online video] – O Level Secondary Chemistry Tuition

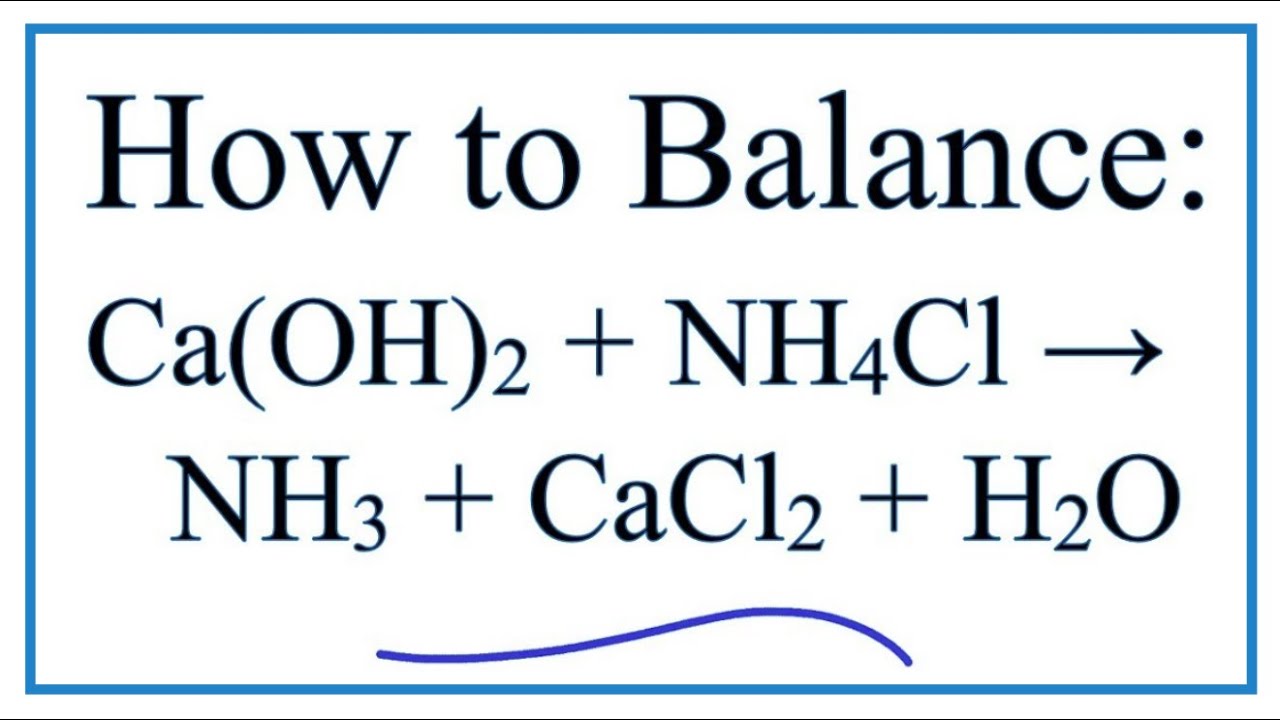
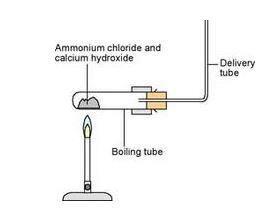
Ammonia, calcium chloride and water are obtained by heating a mixture of ammonium chloride and calcium hydroxide. Write a balanced equation of the reaction.
![Why calcium ions do not form precipitate with aqueous ammonia [online video] – O Level Secondary Chemistry Tuition Why calcium ions do not form precipitate with aqueous ammonia [online video] – O Level Secondary Chemistry Tuition](https://icandochemistry942105908.files.wordpress.com/2021/10/pic-4.jpg?w=1024)
Why calcium ions do not form precipitate with aqueous ammonia [online video] – O Level Secondary Chemistry Tuition
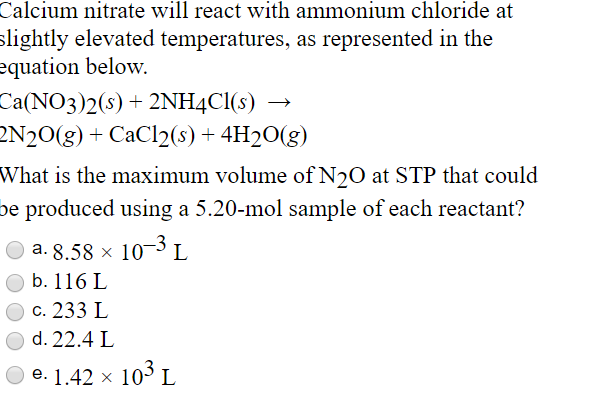
Obtained when 32.6g. of ammonium chloride reacts with calcium hydroxide during the laboratory. - Sarthaks eConnect | Largest Online Education Community