
Urchin-like Bi(OH)SO4 ⋅ H2O fabricated by anodization and its conversion into flower-like Bi2S3 via anion exchange - ScienceDirect

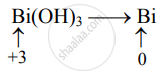
Bi(OH)3 oxidation state @mydocumentary838. find the oxidation number of bismuth in bi(oh)3. - YouTube

Bi(OH)3/PdBi Composite Nanochains as Highly Active and Durable Electrocatalysts for Ethanol Oxidation | Nano Letters

Bi(OH)3 oxidation state @mydocumentary838. find the oxidation number of bismuth in bi(oh)3. - YouTube

SOLVED: Use the rules (in order) to assign oxidation numbers to each of the elements in the compounds below: hydroxylamine NH,OH thiosulfate ion Sz03 bismuth hydroxide Bi(OH)3

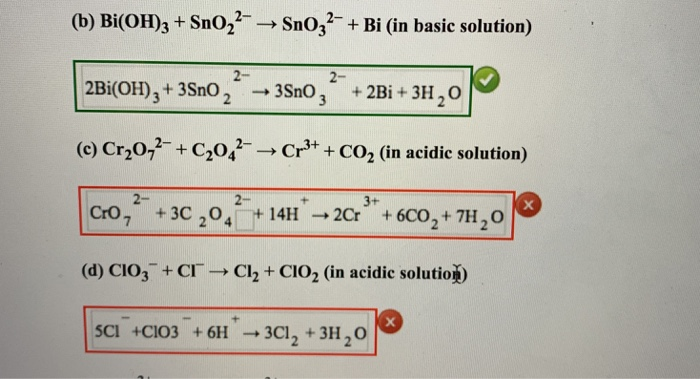
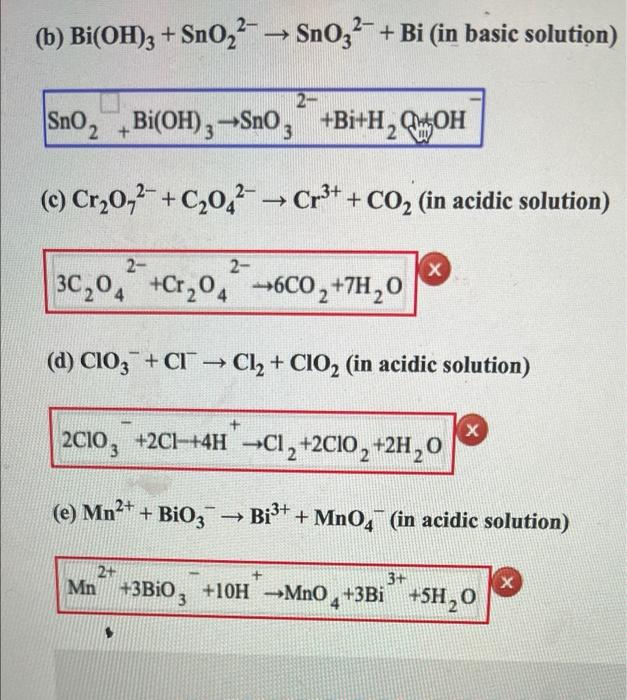
Balance the following redox reactions by ion - electron method:1. MnO4^ - (aq) + I^ - (aq) → MnO2(s) + I2(s) ( in basic medium)2. MnO4^ - (aq) + SO2(g) → Mn^2 + (aq) + HSO4^ - (aq) ( in acidic solution)

Separation and Identification of Bismuth and Tin Ions Experiment 6 Qualitative Analysis. - ppt download

Chapter 4Reactions in Aqueous Solutions. Some typical kinds of chemical reactions: 1.Precipitation reactions: the formation of a salt of lower solubility. - ppt download

Balance the following equation by ion electron method. Zn + NO^ - 3 + OH^ - → ZnO^2 - 2 + NH3 + H2O (alkaline medium)

Biocompatible Bi(OH)3 nanoparticles with reduced photocatalytic activity as possible ultraviolet filter in sunscreens - ScienceDirect



3%20+%20NaOH%20=%20Bi(OH)3%20+%20NaNO3.svg)
![ANSWERED] Use the rules (in order) to assign oxidat... - Physical Chemistry ANSWERED] Use the rules (in order) to assign oxidat... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/50803193-1659119749.0329337.jpeg)
3.svg)


