![When a solution of benzoic acid was titrated with NaOH the pH of the solution when half the acid neutralized was 4.3 . Dissociation constant of the acid is [Use log 2 = 0.3] . When a solution of benzoic acid was titrated with NaOH the pH of the solution when half the acid neutralized was 4.3 . Dissociation constant of the acid is [Use log 2 = 0.3] .](https://haygot.s3.amazonaws.com/questions/1835171_829774_ans_40c76abc129644cfaff5cbb818338569.png)
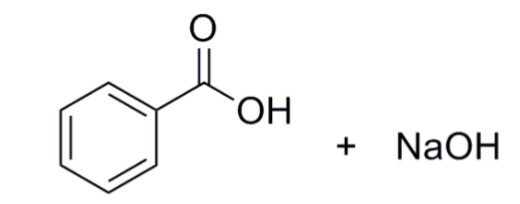
When a solution of benzoic acid was titrated with NaOH the pH of the solution when half the acid neutralized was 4.3 . Dissociation constant of the acid is [Use log 2 = 0.3] .
Choose the correct representation of conductometric titration of benzoic acid vs sodium hydroxide. - Sarthaks eConnect | Largest Online Education Community

Titration curves of benzoic acid for different analyte concentrations... | Download Scientific Diagram
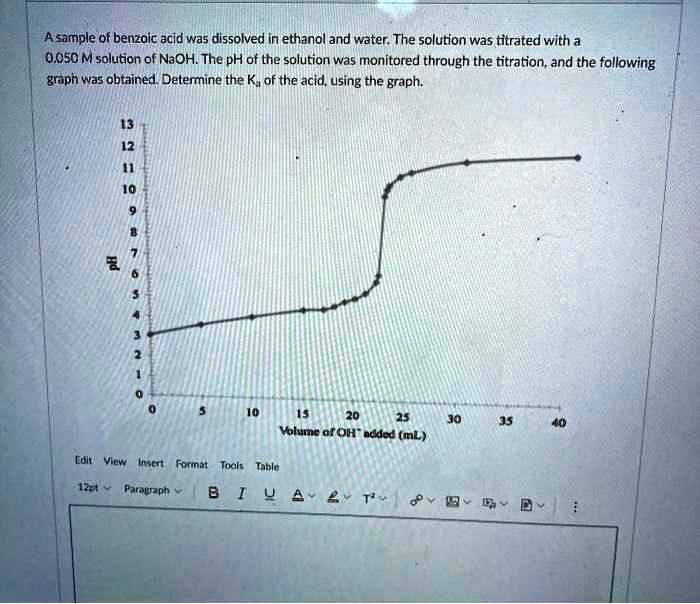
SOLVED: A sample of benzoic acid was dissolved in ethanol and water: The solution was titrated with a 0.O5O M solution of NaOH The pH of the solution was monitored through the

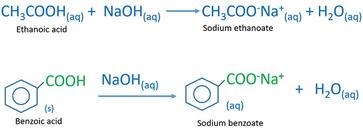
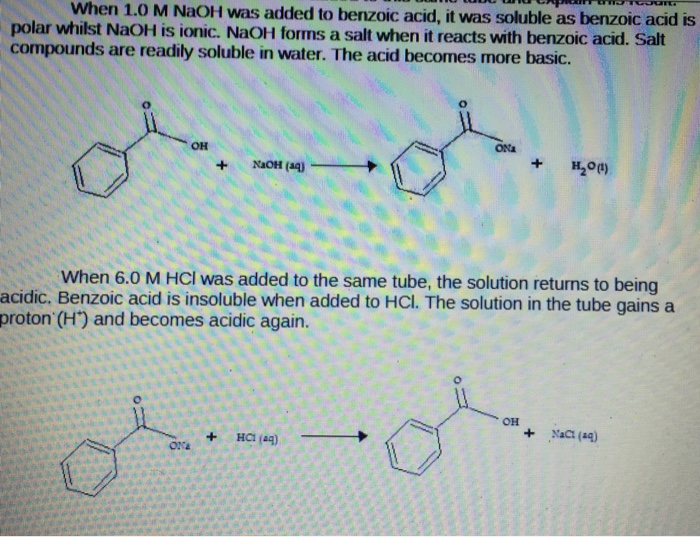
Explain the results for the tube in which 1.0 m naoh was added to benzoic acid. write an equation for this, - Brainly.com

When a solution of benzoic acid was titrated with `NaOH` the `pH` of the solution when half - YouTube
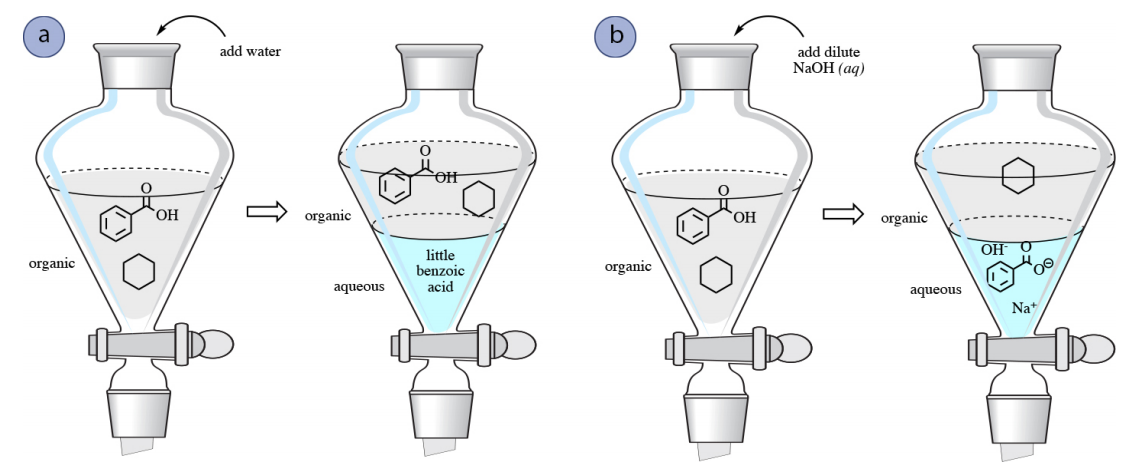
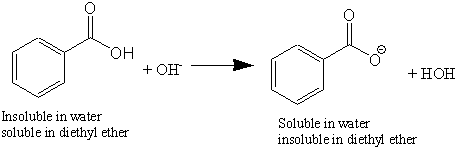
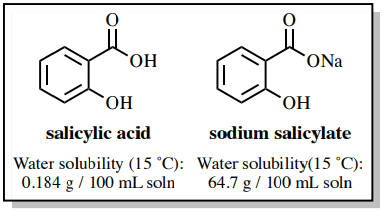
✓ Solved: When benzoic acid ( 5 ) is partitioned between diethyl ether and aqueous sodium hydroxide solution...

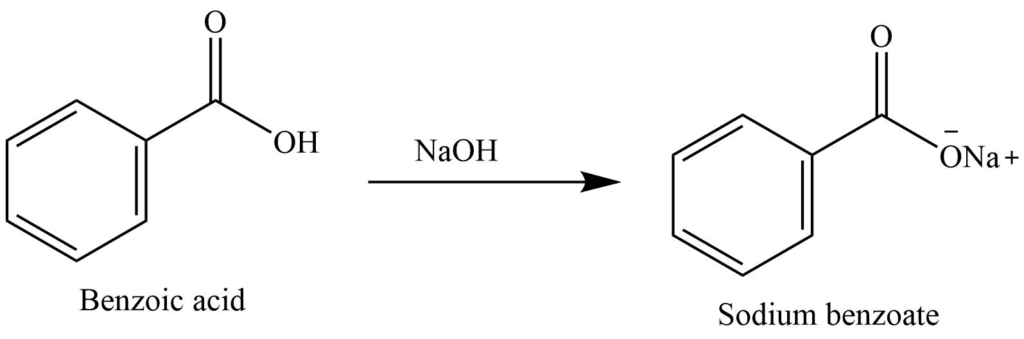
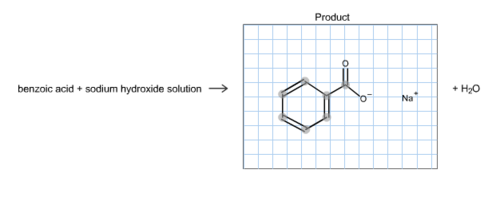
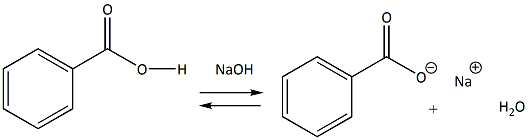
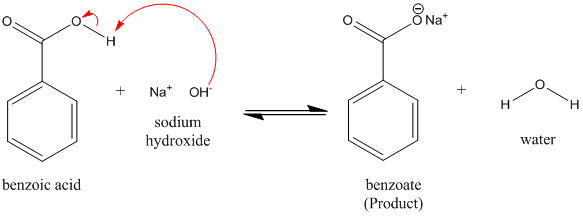
Write the mechanism for the reaction of either benzoic acid or acetic acid with NaOH. Be sure to include all major structures and resonance forms. | Homework.Study.com

SOLVED: A solution of benzoic acid (HC,HsOz) is titrated using sodium hydroxide: The Ka of benzoic acid is 6.4x 101. Write the balanced equation and the net ionic equation. 2.100.0 mL of