Solubility diagram of amorphous Al(OH) 3 as a function of pH at 258C... | Download Scientific Diagram

Annealing induced structural and phase transitions in anodic aluminum oxide prepared in oxalic acid electrolyte - ScienceDirect
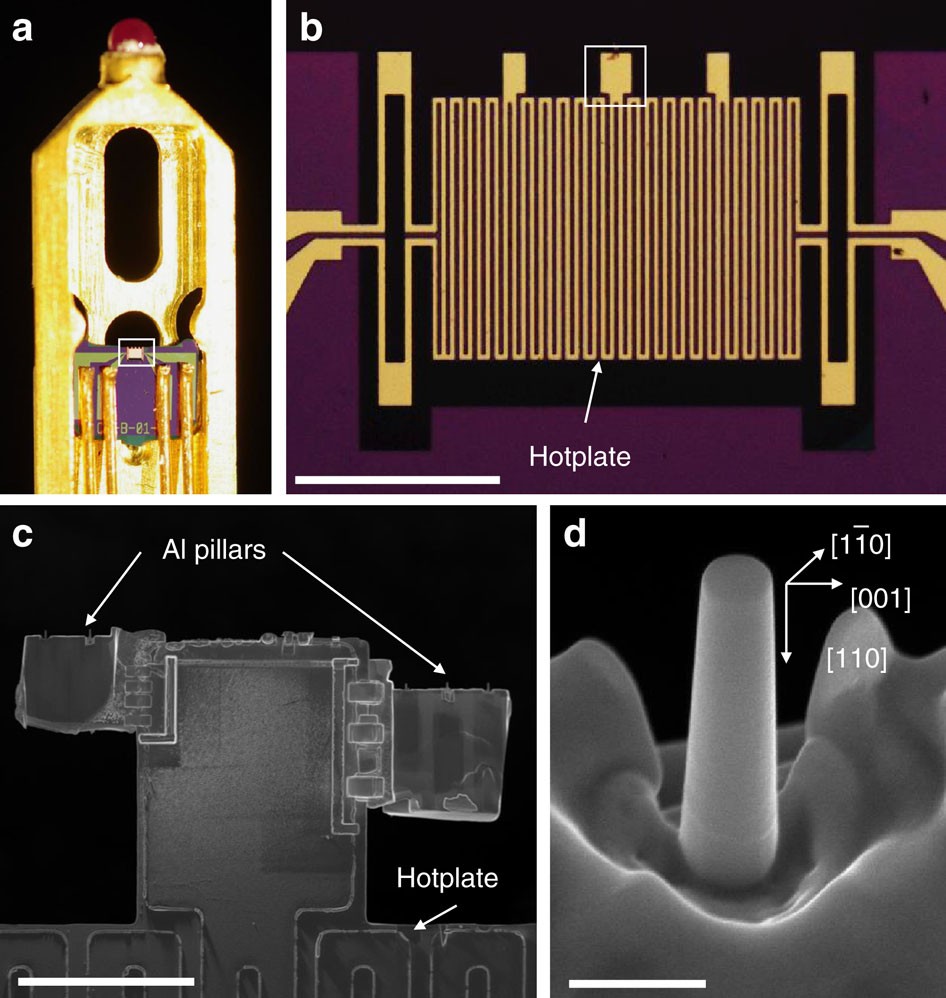
Effect of hydrogen on the integrity of aluminium–oxide interface at elevated temperatures | Nature Communications

The Selective Preparation of an Aluminum Oxide and Its Isomeric C−H-Activated Hydroxide | Journal of the American Chemical Society

Aluminum and aluminum oxide nanomaterials uptake after oral exposure - a comparative study | Scientific Reports
Theoretical aluminum solubility diagram in equilibrium with amorphous... | Download Scientific Diagram

Aluminium solubility as a function of pH. Concentration of aluminium... | Download Scientific Diagram

Solubility diagram of aluminium hydroxide Al(OH) 3 (s) considering only | Download Scientific Diagram

Solubility of Al2O3 in the Na2O−Al2O3−H2O−CH3OH System at (30 and 60) °C | Journal of Chemical & Engineering Data
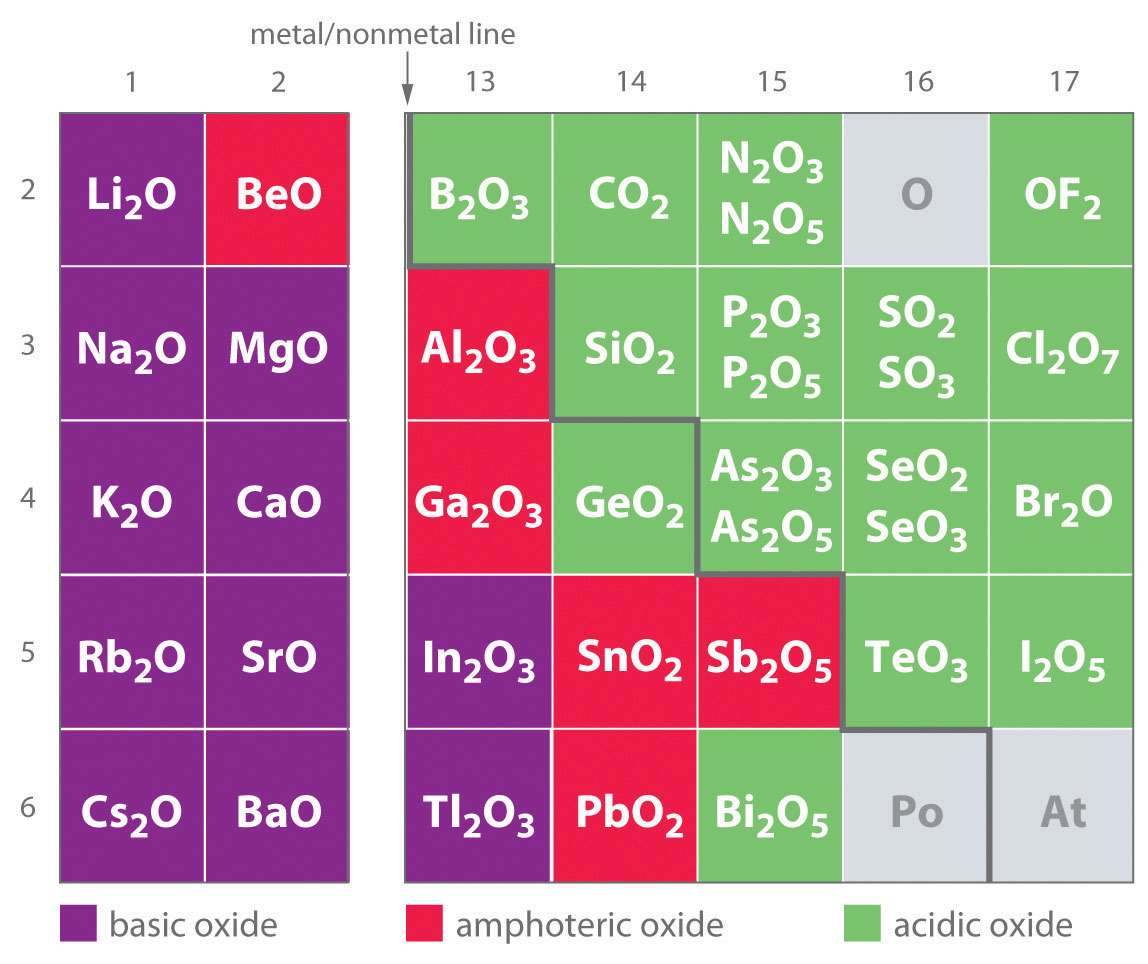
Amphoteric oxides,such as aluminium oxide, are soluble both in strongly acidic and in strongly basic solutions. Dissolution of Al(OH)3 in excess base is just a special case of the effect of complex-ion

Solubility and Modeling of Sodium Aluminosilicate in NaOH–NaAl(OH)4 Solutions and Its Application to Desilication | Industrial & Engineering Chemistry Research