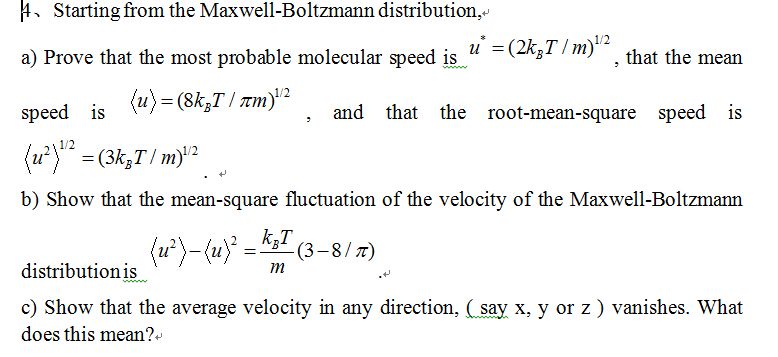
SOLVED: What, according to the Maxwell-Boltzmann distribution, is the proportion of gas molecules having (a) more than, (b) less than the root mean square speed? (c) what are the proportions having speed
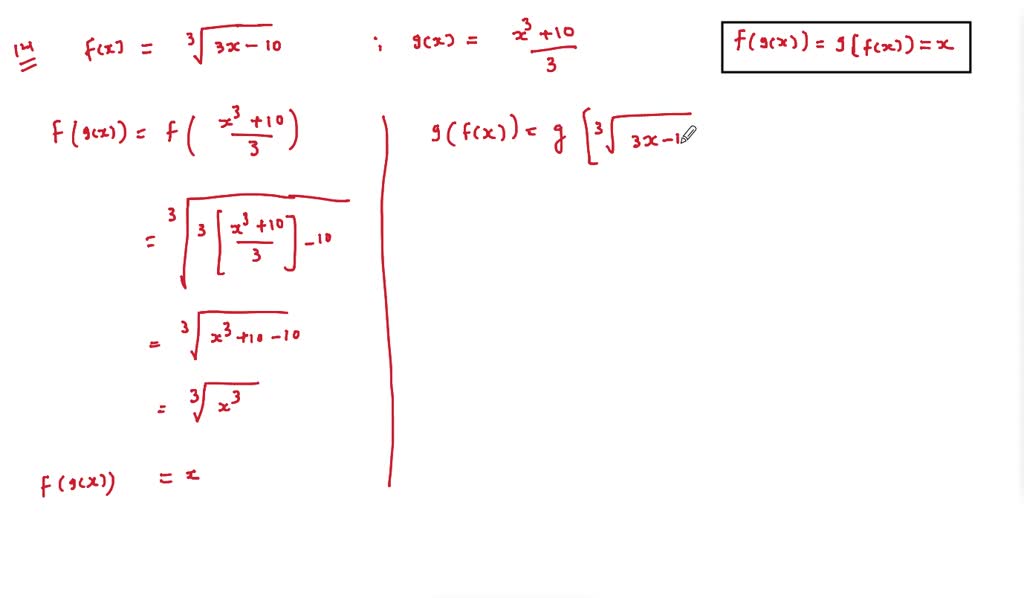
SOLVED:In Exercises 9-14, (a) show that f and g are inverse functions algebraically and (b) use a graphing utility to create a table of values for each function to numerically show that
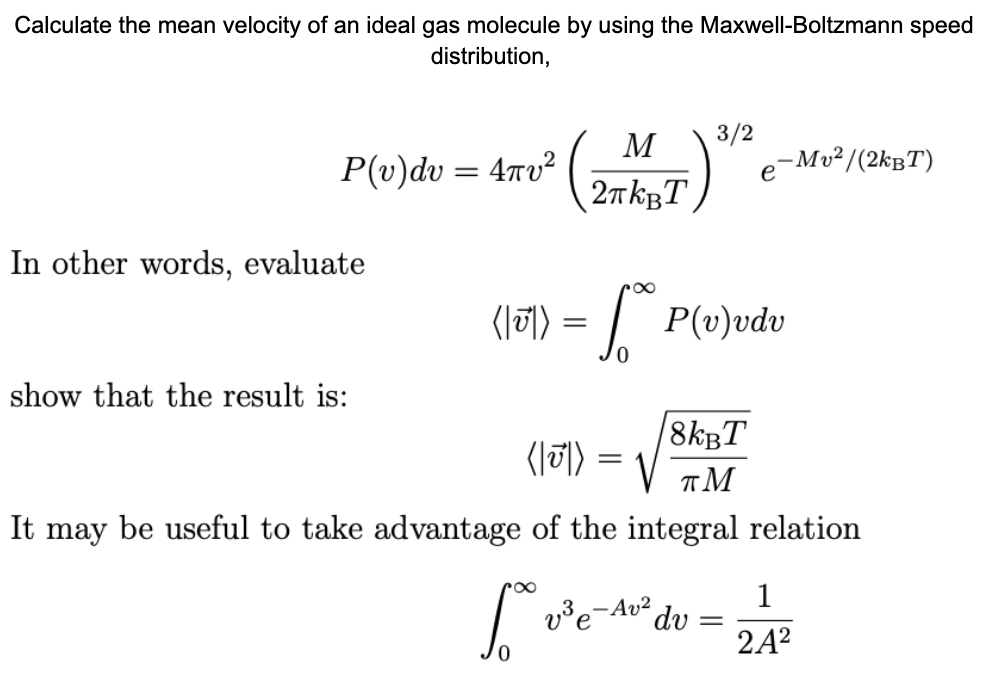
Show that the average speed of gas molecule <ν>= √(8kT/πm). - Sarthaks eConnect | Largest Online Education Community

Ion Solvation Engineering: How to Manipulate the Multiplicity of the Coordination Environment of Multivalent Ions | The Journal of Physical Chemistry Letters

Ion Solvation Engineering: How to Manipulate the Multiplicity of the Coordination Environment of Multivalent Ions | The Journal of Physical Chemistry Letters

SOLVED: The molecular dlameter of CO Is 3.19x 10 " cm: At 600 K and 3 pressure of 200 mm Hg what will the number of molecules colliding per cubic centimeter per

Ion Solvation Engineering: How to Manipulate the Multiplicity of the Coordination Environment of Multivalent Ions | The Journal of Physical Chemistry Letters
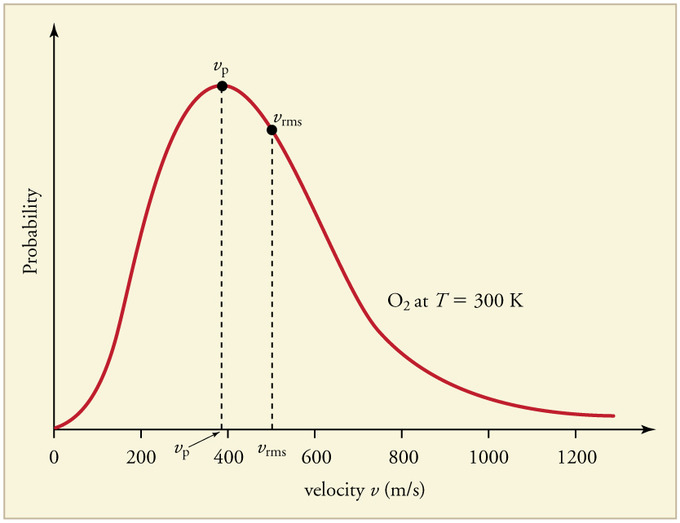
27.3: The Distribution of Molecular Speeds is Given by the Maxwell-Boltzmann Distribution - Chemistry LibreTexts
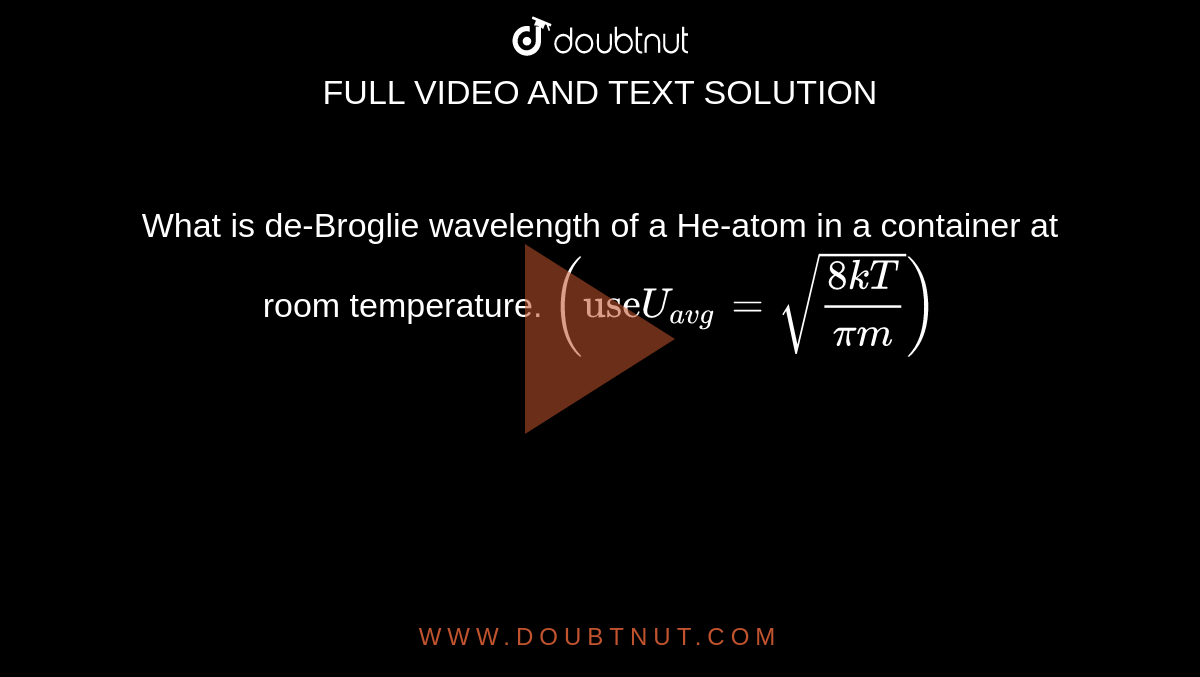
What is de-Broglie wavelength of a He-atom in a container at room temperature. ("use"U(avg)=sqrt((8kT)/(pim)))